Abstract
Background
Syphilis is an infectious disease that is at least discussed to be premalignant. This potential, combined with its general pathological impact, raises the question if syphilis increases mortality in oral cancer patients. The aim of the study was to assess if the five-year survival rates among patients suffering from oral squamous cell carcinoma (OSCC) with (cohort I) and without association with syphilis (cohort II) differ.
Methods
Retrospective clinical data of patients diagnosed with OSCC (International Classification of Diseases [ICD]-10 codes C01–06) within the past 20 years from the access date September 25, 2021 were retrieved from the TriNetX network (TriNetX, Cambridge, Massachusetts, USA) to gain initial cohort 0. Subjects also diagnosed with syphilis (ICD-10 codes A51–53) were assigned to cohort I. Cohort II was comprised of the remaining individuals of cohort 0 by creating a group with the same number of patients as cohort I, and by matching for age and gender. Subsequently, Kaplan-Meier analysis and Cox proportional hazards regression were performed, and risk, odds and hazard ratios were calculated.
Results
Of a total of 73,736 patients in cohort 0, 199 individuals were each assigned to cohort I and II. During the five-year period after tumor diagnosis, 39 and 30 patients in cohort I and II died. The five-year survival probabilities did not significantly differ between the cohorts (I vs. II = 74.19% vs. 75.01%; p = 0.52; Log-Rank test), nor the risk of dying (I vs. II = 19.6% vs. 15.08%; risk difference = 4.52%; p = 0.23). The calculated risk, odds and hazard ratios were 1.3 (95% confidence interval [CI] = 0.84; 2.00), 1.37 (95% CI = 0.81; 2.31) and 1.17 (95% CI = 0.73; 1.88), respectively.
Conclusions
The obtained results indicate that the survival rate of individuals with OSCC might not be negatively influenced if syphilis is present/associated. However, the results need to be interpreted cautiously due to limitations related to the retrospective approach, especially as data on the tumor staging were not accessible.
Trial registration
Due to the retrospective nature of the study, no registration was necessary.
Keywords: Oral malignant neoplasia, Oral squamous cell carcinoma, Syphilis, Premalignant condition, Mortality, Survival rate, Multi-center data
Background
Syphilis is an infectious disease caused by the bacteria Treponema pallidum, subsp. pallidum. It is mostly transmitted sexually [1]. All stages of the disease can cause maxillofacial, especially oral, manifestations [2]. Even though the authors of the recent World Health Organization (WHO) classification did not find sufficient evidence to classify syphilis as an oral potentially malignant disorder (OPMD) [3], it was at least controversially discussed whether oral lesions caused by syphilis, as well as the disease itself, could be associated with an augmented risk of developing different malignant neoplasia, inter alia oral squamous cell carcinoma (OSCC) [4–9]. Among the key alterations fundamental to cancer cell development [10], syphilis might especially establish a tumor-promoting inflammation and trigger sustaining proliferative signaling. Besides the fact that the presence of syphilis might enhance the risk of developing tumors, it also acts as a co-morbidity, regardless of its questionable malignant character. Both features might potentially impede outcomes in patients with OSCC. Accordingly, the purpose of the present study was to analyze if the five-year survival rates among patients with oral cancer with and without association with syphilis differed. In the recent literature, this question has not yet been addressed. It was hypothesized that the survival rate of subjects suffering from OSCC associated with syphilis was significantly higher compared to individuals with oral cancer without syphilis.
Regardless of its rising incidence within the last decades in specific cohorts in high-income countries, syphilis is still a relatively rare disease [1]. The TriNetX Global Health Research Network (TriNetX, Cambridge, Massachusetts, USA) was chosen to retrieve related retrospective data, as it provides access to a significant number of medical records. TriNetX is a database that includes clinical data from more than 120 health care organizations (HCOs) from 19 countries. Its purpose is to enable HCOs, contract research institutes and biopharmaceutical companies to access and exchange longitudinal clinical data, and to provide state-of-the-art analytics. By September 2021, TriNetX had collected electronic medical records from more than 250 million patients. It has previously been used to research medical topics of worldwide interest, including the coronavirus disease 2019 (COVID-19) pandemic [11, 12].
Patients and methods
Data acquisition, inclusion and exclusion criteria
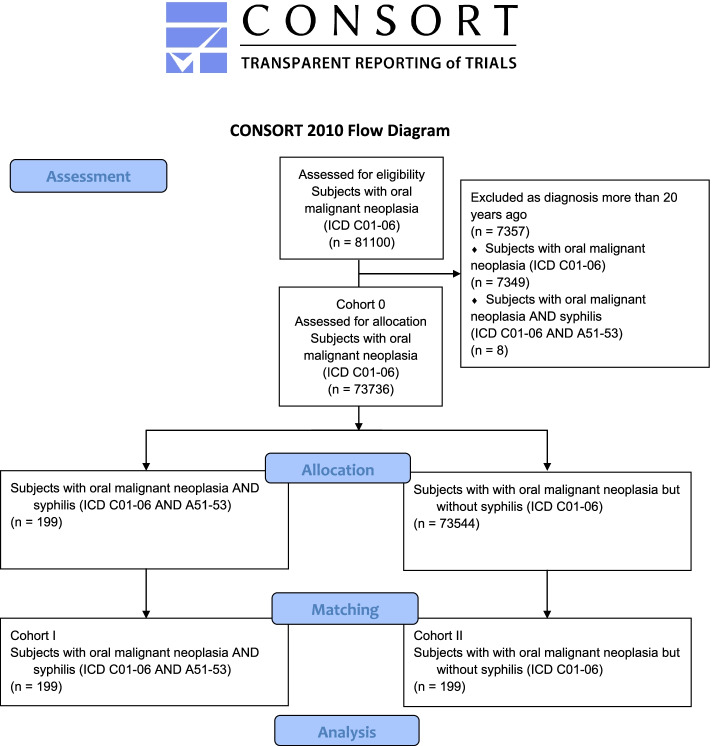
The TriNetX network was accessed on Saturday, September 25, 2021; whereby the eligibility period was limited to the previous 20 years from the access date to take account of recent developments in the diagnosis and therapy of OSCC. The database was searched for patients who were diagnosed with OSCC (International Classification of Diseases [ICD]-10 codes C01–06) between five and 20 years before the access date. Subjects with malignant odontogenic tumors or neoplasia of the lips, salivary glands, tonsillae, oro-, naso and hypopharynx, recessus piriformis, as well as tumors located in other areas different from the oral cavity (ICD-10 codes C00, 07–14, 41.02–41.1, 43 and 44) were not included. The obtained cohort 0 was then tested for diagnosis with early, late or not further specified syphilis (ICD-10 codes A51–53). Patients with syphilis connata (ICD-10 code A50) were not included in the analysis. In order to mitigate confounder bias via propensity score, stratified and balanced sub-cohorts across current age, age at tumor diagnosis and gender distribution were retrieved from the initial cohorts. One-to-one matching was applied in order to replicate randomized conditions as closely as possible by obtaining cohorts with similar covariate distributions. All individuals diagnosed with both ICD-10 codes C01–06 and A51–53 were assigned to cohort I (patients suffering from OSCC and syphilis). Cohort II (subjects with OSCC, but without syphilis) was subsequently obtained from the remaining individuals within cohort 0, and by matching as shown in the CONSORT flow chart (Fig. 1). The final cohorts were furthermore checked for human papillomavirus (HPV) and human immunodeficiency virus (HIV) diagnoses. HPV was diagnosed by means of testing for HPV-DNA in tumor samples, whereas HIV diagnoses were based on serological testing (enzyme-linked immunosorbent assay [ELISA] and HIV antibody test).
Fig. 1.
Modified CONSORT flow chart
Data analysis
After defining the primary outcome as “death”, Kaplan-Meier survival analysis and Cox proportional hazards regression were performed, and risk ratios (RR), odds ratios (OR), as well as hazard ratios (HR), were calculated for the respective cohorts. The evaluation was limited to a period of 5 years after cancer diagnosis as patients are considered to be healed in the case of absence of OSCC and metastases after the respective time. Statistical analysis was performed applying the Log-Rank test, whereby p ≤ 0.05 was defined as a significance threshold.
Results
Assessment, allocation and matching
A total eligible number of 81,100 patients from 65 health care organizations from eleven countries with OSCC (ICD-10 codes C01–06) were retrieved from the database. The number of subjects who were excluded due to diagnosis with either ICD-10 codes C01–06 or A51–53 over the 20 years before the access date were eight (for OSCC and syphilis) and 7349 (for OSCC, but no syphilis). As a result, a total of 73,736 individuals with OSCC were available for allocation to cohort 0. Of those, 199 patients were also diagnosed with syphilis (ICD-10 codes A51–53) and assigned to cohort I (females: 44 [22.11%]; males: 155 [77.89%]; mean(±standard deviation) current age(±standard deviation) = 62.79 ± 13.52 years; mean age at diagnosis = 57.51 ± 13.64 years). The same number of subjects were assigned by matching of both groups to cohort II, as explained above (females: 43 [21.61%]; males: 156 [78.39%]; mean current age = 62.90 ± 13.31 years; mean age at diagnosis = 57.61 ± 13.43 years). The groups did not differ significantly in gender distribution or age (p = 0.90 and 0.93; Log-Rank test). The obtained propensity score was 0.98. Table 1 shows the patient characteristics of both cohorts before and after matching.
Table 1.
Patient characteristics before and after matching of cohorts I (ICD-10 codes C01–06 and A51–53) and II (ICD-10 codes C01–06 without A51–53)
| Patients (n) | Before matching | After matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Cohort I | Cohort II | p- value | Standardized mean difference | Cohort I | Cohort II | p-value | Standardized mean difference | |
| Total | 199 | 73,544 | 199 | 199 | ||||
| Male | 155 (77.89%) | 49,015 (66.65%) | < 0.05 | 0.2531 | 155 (77.89%) | 156 (78.39%) | 0.90 | 0.0121 |
| Female | 44 (22.11%) | 24,488 (33.25%) | < 0.05 | 0.2519 | 44 (22.11%) | 43 (21.61%) | 0.90 | 0.0121 |
| Mean current age (years) | 62.79 | 68.18 | < 0.05 | 0.3956 | 62.79 | 62.90 | 0.93 | 0.0082 |
| Standard deviation | 13.52 | 13.73 | 13.52 | 13.31 | ||||
| Minimum | 7 | 0 | 7 | 7 | ||||
| Maximum | 90 | 90 | 90 | 90 | ||||
| Mean age at diagnosis | 57.51 | 61.94 | < 0.05 | 0.3207 | 57.51 | 57.61 | 0.93 | 0.0077 |
| Standard deviation | 13.64 | 13.97 | 13.64 | 13.43 | ||||
| Minimum | 4 | 0 | 4 | 4 | ||||
| Maximum | 90 | 90 | 90 | 90 | ||||
Percentage refers to gender distribution within the respective cohorts. P-value refers to comparison between both cohorts (Log-Rank test)
Abbreviations: ICD International Classification of Diseases
Patient survival
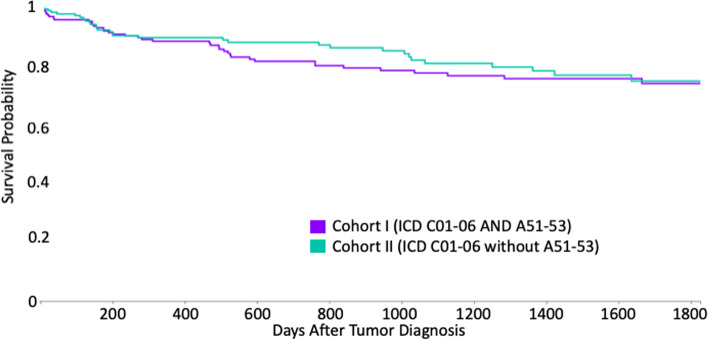
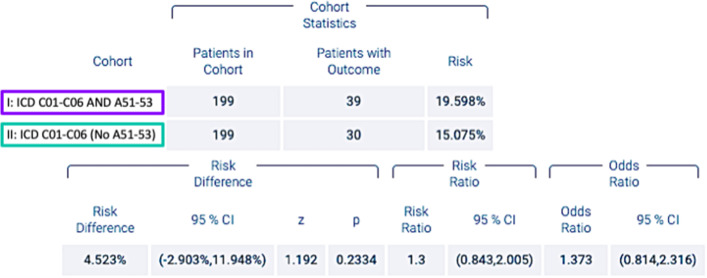
During the five-year period after diagnosis of OSCC, 39 patients in cohort I died, whereas 30 individuals in cohort II passed away, which corresponds to risks of death of 19.59 and 15.07%, respectively. The difference between the cohorts was not statistically significant (p = 0.233; Log-Rank test). Accordingly, the survival probability at the end of the time window was 74.19 and 75.01%, respectively, for subjects among cohorts I and II (Fig. 2). In addition, no statistical significance was found (p = 0.52). The related RR, OR and HR were 1.3 (95% confidence interval (CI) lower: 0.84 and upper: 2.00), 1.37 (95% CI lower: 0.81 and upper: 2.31) and 1.17 (95% CI = 0.73; 1.88), respectively, as shown in Fig. 3.
Fig. 2.
Kaplan-Meier survival curve of cohorts I (ICD-10 codes C01–06 and A51–53) and II (ICD-10 codes C01–06 without A51–53)
Fig. 3.
Risk of death, risk ratios and odds ratios of cohorts I (ICD-10 codes C01–06 and A51–53) and II (ICD-10 codes C01–06 without A51–53)
HPV and HIV infection
Both cohorts were negatively tested for difference in distribution to HPV diagnoses (p > 0.05). However, significantly more cases of HIV infection were present in cohort I (n = 56; females: 10 and males: 46) compared to cohort II (p < 0.05). Therefore, subcohorts that were matched for the presence of HIV, as well as for gender and age distribution within the infected, and which consisted of 55 patients each, underwent statistical testing again. In accordance with the results presented above, the risks of death and survival probabilities did not differ significantly between those subcohorts (p > 0.05).
Discussion
The aim of the present work was to evaluate if the five-year survival rates among patients suffering from OSCC and syphilis differed from individuals with oral cancer, but without syphilis. This study was the first to address this question by retrospectively evaluating data from multiple centers to investigate larger cohorts. Different from the hypothesis expressed in the introduction, it was found that the survival rates of individuals with OSCC did not differ significantly if syphilis was parallelly diagnosed, compared to subjects without syphilis. At least, OR was found to be 1.3, which indicates a trend in accordance with the hypothesis. Furthermore, the survival curves of the investigated cohorts show intermediary discrepancies before they align at the end of the time window (Fig. 2).
The obtained results certainly support the recent WHO classification, according to which syphilis in no longer seen as an OPMD due to the lack of sufficient evidence of any premalignant character [3]. Even authors who discussed the role of the disease in oral cancer, and attributed syphilis with an etiologic factor in tumorigenesis, described it as one of relatively moderate impact [5, 6]. Despite the fact that syphilis itself acts as a co-morbidity, especially in its early stages, it can be sufficiently and cost-effectively treated with antibiotics [13]. The availability of a prompt curative treatment, at least in mid- and high-income countries, might limit the pathological potential of syphilis in this regard. However, the retrieved results need to be discussed regarding limitations that stem from the specific design of the present study. Specifically, the TriNetX database can be searched for patients with specific diagnoses by using the respective ICD-10 codes, which implies that oral malignant neoplasia are classified according to their localization. The vast majority of these tumors were supposedly OSCCs, as the most frequent malignant tumor of the oral cavity [5, 14, 15]. In addition, certain malignant entities of odontogenic, lymphatic or osseous origin, for example, are classified separately within the ICD-10, and have therefore been excluded. Nevertheless, patients suffering from different subtypes of OSCCs or even other rare oral malignant neoplasia (e.g., oral malignant melanoma) might have been included in the investigated cohorts. This could have influenced the obtained results as different malignant (sub-)entities show varying biological and pathological features, such as proliferation rate or specific risk of developing metastases [16]. Furthermore, different characteristics have been identified to influence the survival rates of patients with oral malignant neoplasia, even though other features are still discussed controversially [10]. Localization and the staging of the tumor disease according to the Union Internationale Contre le Cancer (UICC), including clinical, histological/pathological and molecular features, as well as the applied therapies, have especially been found to significantly influence patient probability of survival [5, 17–23]. Smoking behavior, alcohol abuse and the presence of human papilloma virus are not only etiological factors, but also factors that impact the prognosis of patients with oral cancer [23, 24]. Ideally, the investigated cohorts would have been matched for UICC stage. From the TriNetX network, no respective data was available. However, features related to the lacking information might have influenced the mortality of patients with OSCC/syphilis within the investigated cohorts. In contrast, matching of the compared cohorts for age and gender might have levelled out the differences in distribution of these variables, at least to a certain extent. Due to the limited availability of certain data, the percentage of patients of cohort II who tested negatively for syphilis also could not be assessed. Supposedly, testing was not carried out routinely, at least in cases without respective suspicion. Therefore, uncertainty remains that undetected cases of syphilis might have been included in cohort II. Despite the lack of the mentioned information, the quality of the data retrieved from the TriNetX database can be classified as high. The database even matches the strict conditions of the National COVID Cohort Collaborative N3C, which was formed to accelerate the understanding of SARS-CoV-2, and of which TriNetX recently became a part.
To overcome the aforementioned limitations, future research might consider using a prospective approach applying standardized therapies, including routine testing for syphilis, to evaluate if the presented results can be confirmed thus far. In addition, histological/pathological and molecular data may be included into the analysis. Despite this, the relatively low incidence of syphilis remains problematic regarding an appropriate prospective study design [1].
Conclusions
Syphilis is no longer classified as OPMD within the recent WHO classification [3], even though its premalignant potential has at least been discussed. Despite its questionable role as a contributor to oral cancer, and its impact as a co-morbidity, the present study did not show a negative influence of syphilis on the five-year survival rate of patients with OSCC, compared to patients without syphilis. However, these results should be cautiously interpreted due to limitations related to the applied approach.
Acknowledgements
Not applicable.
Abbreviations
- ELISA
Enzyme-linked immunosorbent assay
- HCO
Heath care organization
- HIV
Human immunodeficiency virus
- HPV
Human papillomavirus
- HR
Hazard ratio
- ICD
International Classification of Diseases
- OR
Odds ratio
- OPMD
Oral potentially malignant disorder
- OSCC
Oral squamous cell carcinoma
- RR
Risk ratio
- UICC
Union Internationale Contre le Cancer
- WHO
World Health Organization
Authors’ contributions
M.H. and S.P. conceived and designed the study. All authors were involved in data acquisition, analysis and interpretation, as well as creation of Figs. 1, 2, 3 and Table 1.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by TRR295 and KFO339.
Availability of data and materials
The datasets used and analyzed can be retrieved from the TriNetX network (https://trinetx.com). Public access to the database is closed. If no access is available, the datasets can be retrieved from the corresponding author based on reasonable request.
Declarations
Ethics approval and consent to participate
Administrative access to the database was granted by TriNetX. All methods were carried out in accordance with relevant guidelines and regulations. All HCOs from which data were transmitted to TriNetX obtained informed consent in written from all patients and/or their legal guardians. TriNetX is compliant with the Health Insurance Portability and Accountability Act (HIPAA), the US federal law which protects the privacy and security of healthcare data. TriNetX is certified to the ISO 27001:2013 standard and maintains an Information Security Management System (ISMS) to ensure the protection of the healthcare data it has access to and to meet the requirements of the HIPAA Security Rule. Any data displayed on the TriNetX Platform in aggregate form, or any patient level data provided in a data set generated by the TriNetX Platform, only contains de-identified data as per the de-identification standard defined in Section §164.514(a) of the HIPAA Privacy Rule. The process by which the data is de-identified is attested to through a formal determination by a qualified expert as defined in Section §164.514(b) [1] of the HIPAA Privacy Rule. This formal determination by a qualified expert, refreshed in December 2020, supersedes the need for TriNetX’s previous waiver from the Western Institutional Review Board (IRB). The TriNetX network contains data provided by participating Healthcare Organizations (HCOs), each of which represents and warrants that it has all necessary rights, consents, approvals and authority to provide the data to TriNetX under a Business Associate Agreement (BAA), so long as their name remains anonymous as a data source and their data are utilized for research purposes. The data shared through the TriNetX Platform are attenuated to ensure that they do not include sufficient information to facilitate the determination of which HCO contributed which specific information about a patient (https://trinetx.com/trinetx-publication-guidelines/).
Access to the database is closed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hook EW., 3rd Syphilis. Lancet. 2017;389:1550–1557. doi: 10.1016/S0140-6736(16)32411-4. [DOI] [PubMed] [Google Scholar]
- 2.Hertel M, Matter D, Schmidt-Westhausen AM, Bornstein MM. Oral syphilis: a series of 5 cases. J Oral Maxillofac Surg. 2014;72:338–345. doi: 10.1016/j.joms.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Warnakulasuriya S, Kujan O, Aguirre-Urizar JM, Bagan JV, Gonzalez-Moles MA, Kerr AR, et al. Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO collaborating Centre for Oral Cancer. Oral Dis. 2021;27:1862–1880. doi: 10.1111/odi.13704. [DOI] [PubMed] [Google Scholar]
- 4.Binnie WH, Rankin KV, Mackenzie IC. Etiology of oral squamous cell carcinoma. J Oral Pathol. 1983;12:11–29. doi: 10.1111/j.1600-0714.1983.tb00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Sciubba JJ. Oral cancer. The importance of early diagnosis and treatment. Am J Clin Dermatol. 2001;2:239–251. doi: 10.2165/00128071-200102040-00005. [DOI] [PubMed] [Google Scholar]
- 6.Castigliano SG. Syphilis and oral cancer. Ann Otol Rhinol Laryngol. 1955;64:608–616. doi: 10.1177/000348945506400228. [DOI] [PubMed] [Google Scholar]
- 7.Michalek AM, Mahoney MC, McLaughlin CC, Murphy D, Metzger BB. Historical and contemporary correlates of syphilis and cancer. Int J Epidemiol. 1994;23:381–385. doi: 10.1093/ije/23.2.381. [DOI] [PubMed] [Google Scholar]
- 8.Rahima S, Riyaz N, Latheef EN, Shyni PM. Squamous cell carcinoma on a syphilitic gumma: A unique presentation. Indian J Sex Transm Dis AIDS. 2015;36:89–91. doi: 10.4103/0253-7184.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickenson AJ, Currie WJ, Avery BS. Screening for syphilis in patients with carcinoma of the tongue. Br J Oral Maxillofac Surg. 1995;33:319–320. doi: 10.1016/0266-4356(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Sasahira T, Kirita T. Hallmarks of Cancer-related newly prognostic factors of Oral squamous cell carcinoma. Int J Mol Sci. 2018;19:2413–34. [DOI] [PMC free article] [PubMed]
- 11.Mura C, Preissner S, Nahles S, Heiland M, Bourne PE, Preissner R. Real-world evidence for improved outcomes with histamine antagonists and aspirin in 22,560 COVID-19 patients. Signal Transduct Target Ther. 2021;6:267. doi: 10.1038/s41392-021-00689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seeland U, Coluzzi F, Simmaco M, Mura C, Bourne PE, Heiland M, et al. Evidence for treatment with estradiol for women with SARS-CoV-2 infection. BMC Med. 2020;18:369. doi: 10.1186/s12916-020-01851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morales-Munera CE, Fuentes-Finkelstein PA, Vall MM. Update on the diagnosis and treatment of syphilis. Actas Dermosifiliogr. 2015;106:68–69. doi: 10.1016/j.ad.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Sellars SL. Epidemiology of oral cancer. Otolaryngol Clin N Am. 1979;12:45–55. doi: 10.1016/S0030-6665(20)32492-0. [DOI] [PubMed] [Google Scholar]
- 15.Scully C, Bagan J. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009;15:388–399. doi: 10.1111/j.1601-0825.2009.01563.x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. doi: 10.1177/014556130608500201. [DOI] [PubMed] [Google Scholar]
- 17.Rogers SN, Brown JS, Woolgar JA, Lowe D, Magennis P, Shaw RJ, et al. Survival following primary surgery for oral cancer. Oral Oncol. 2009;45:201–211. doi: 10.1016/j.oraloncology.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Braakhuis BJ, Bloemena E, Leemans CR, Brakenhoff RH. Molecular analysis of surgical margins in head and neck cancer: more than a marginal issue. Oral Oncol. 2010;46:485–491. doi: 10.1016/j.oraloncology.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Graveland AP, Golusinski PJ, Buijze M, Douma R, Sons N, Kuik DJ, et al. Loss of heterozygosity at 9p and p53 immunopositivity in surgical margins predict local relapse in head and neck squamous cell carcinoma. Int J Cancer. 2011;128:1852–1859. doi: 10.1002/ijc.25523. [DOI] [PubMed] [Google Scholar]
- 20.Grimm M. Prognostic value of clinicopathological parameters and outcome in 484 patients with oral squamous cell carcinoma: microvascular invasion (V+) is an independent prognostic factor for OSCC. Clin Transl Oncol. 2012;14:870–880. doi: 10.1007/s12094-012-0867-2. [DOI] [PubMed] [Google Scholar]
- 21.Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130–137. doi: 10.1016/S1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 22.Moro JDS, Maroneze MC, Ardenghi TM, Barin LM, Danesi CC. Oral and oropharyngeal cancer: epidemiology and survival analysis. Einstein (Sao Paulo) 2018;16:eAO4248. doi: 10.1590/s1679-45082018ao4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanoni DK, Montero PH, Migliacci JC, Shah JP, Wong RJ, Ganly I, et al. Survival outcomes after treatment of cancer of the oral cavity (1985-2015) Oral Oncol. 2019;90:115–121. doi: 10.1016/j.oraloncology.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lajer CB, von Buchwald C. The role of human papillomavirus in head and neck cancer. APMIS. 2010;118:510–519. doi: 10.1111/j.1600-0463.2010.02624.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed can be retrieved from the TriNetX network (https://trinetx.com). Public access to the database is closed. If no access is available, the datasets can be retrieved from the corresponding author based on reasonable request.