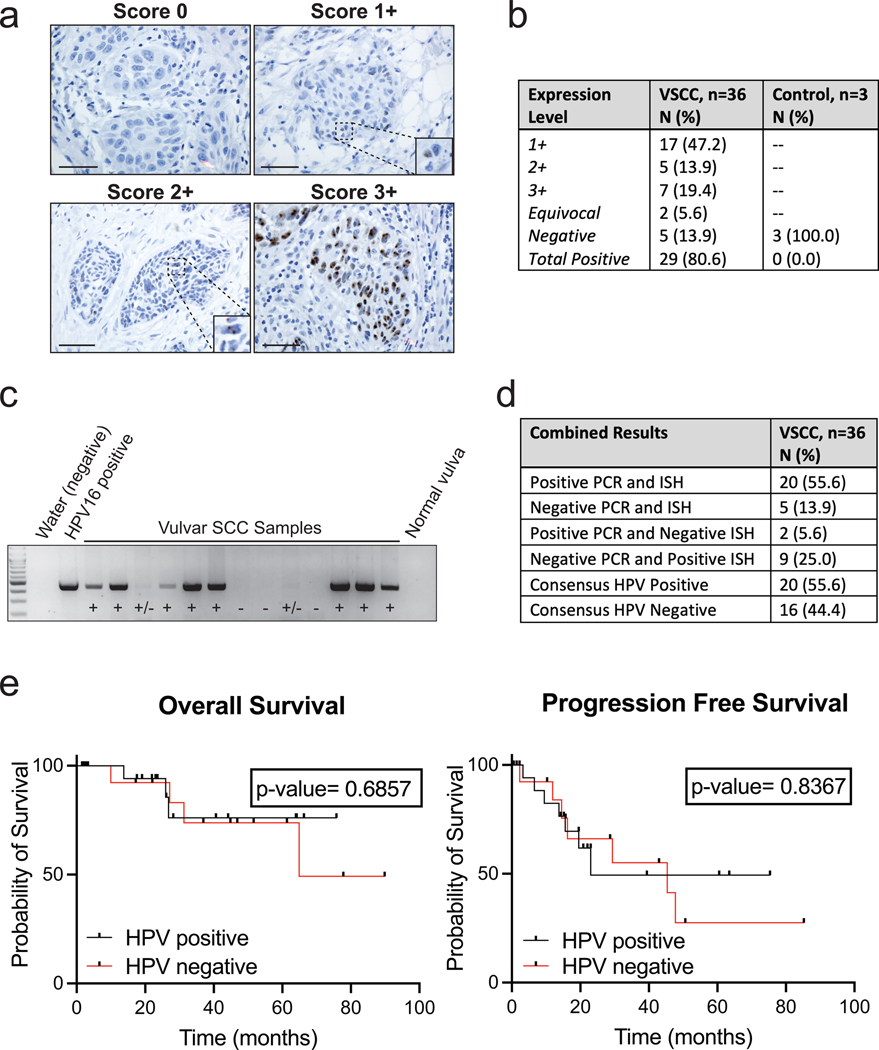
Figure 1. Determination of Presence or Absence of Human Papillomavirus for Vulvar Squamous Cell Carcinoma.
a. Representative images of RNA HPV-ISH results separated by score. Bar = 50 μm
b. Expression scores for HPV-ISH for VSCC and control samples.
c. Representative image of HPV PCR. Positive control represents 10 pg of the HPV-16 added to the outer PCR reaction. Negative controls used include water and a normal vulvar tissue sample without history of HPV infection or neoplasm.
d. PCR and HPV-ISH comparison results.
e. Kaplan–Meier curves for overall survival and progression-free survival by HPV-final consensus status and compared by log-rank test with a p-value <0.05 considered significant.