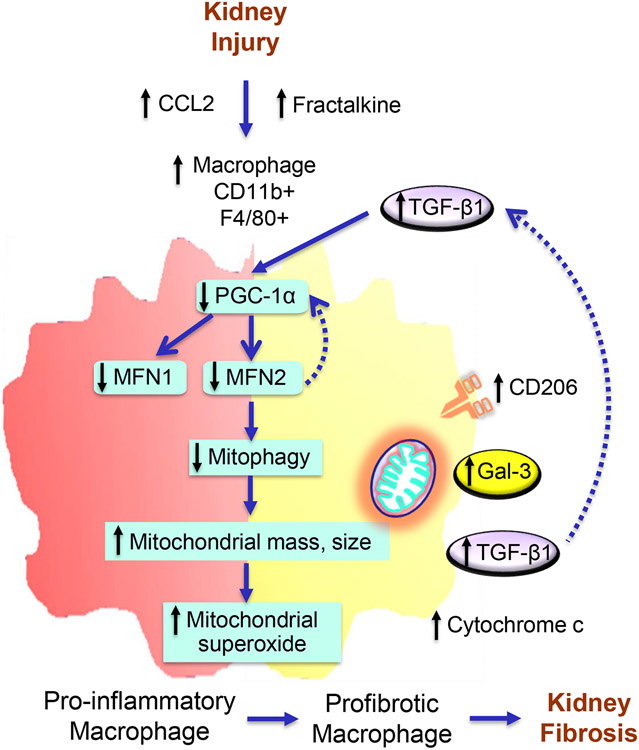
Figure 10. Proposed model of Mitofusin 2-dependent regulation of macrophage switch during kidney fibrosis.
Kidney injury-induced increases in secretion of chemokines, C-C motif chemokine ligand 2 (CCL2), and CX3CL1 favor the recruitment of CCR2 and CX3CR1 expressing pro-inflammatory and anti-inflammatory monocytes/macrophages, respectively, into the kidney. Impaired mitochondrial biogenesis in macrophages after kidney injury resulted in a decrease in the expression of MFN1 and MFN2. Reduced expression of Mitofusin (MFN)-2 but not MFN1 in macrophages following kidney injury contributes to the impairment of mitophagy leading to hyperaccumulation of dysfunctional mitochondria with increased superoxide production and increased circulating plasma and urinary cytochrome c, a mitochondrial damage-related marker. Mitochondrial dysfunction and oxidative stress promote the switching over of pro-inflammatory to profibrotic phenotype with higher polarization towards CD206, galectin-3 (Gal-3), and TGF-β1 expressing profibrotic/M2 macrophages and the progression of kidney fibrosis. TGF-β1, in turn, downregulates the expression of PGC-1α and suppresses MFN1 and MFN2 expression. Deficiency of MFN2 but not MFN1, therefore promotes kidney fibrosis by downregulating mitochondrial biogenesis and mitophagy in macrophages.