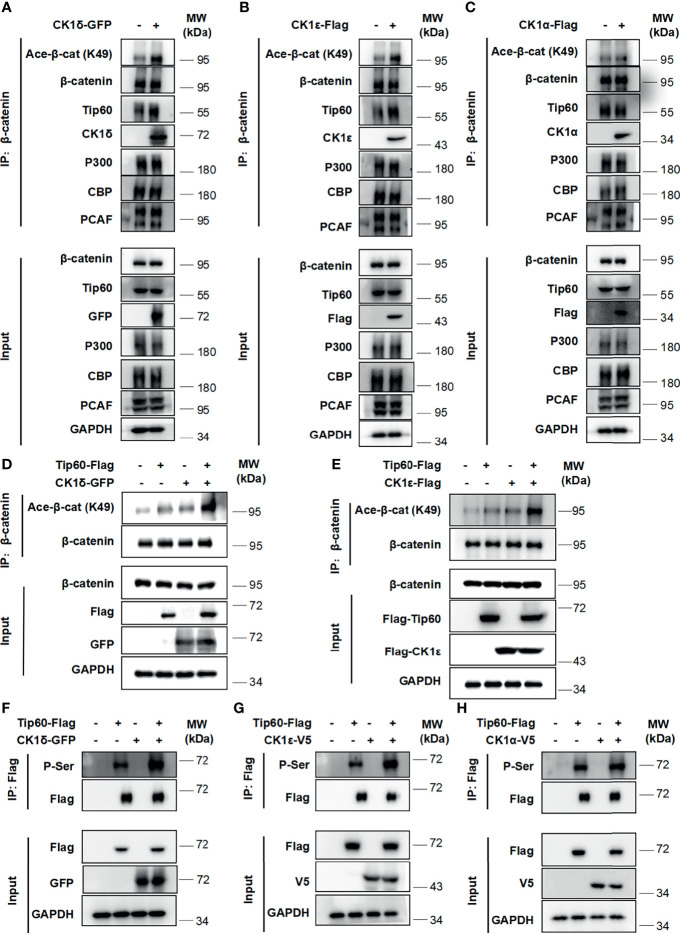
Figure 2.
CK1δ/ϵ promotes β-catenin acetylation through enhancing the association of β-catenin with Tip60. (A–C) HEK293T cells were transfected with CK1δ-GFP, CK1ϵ-Flag and CK1α-Flag expression plasmids, and cell lysates were immunoprecipitated with anti-β-catenin agarose beads. The interaction between β-catenin and some histone acetyltransferases (Tip60, PCAF, p300, and CBP) was detected by immunoblotting. (D, E) HEK293T cells were transfected with control vector or Tip60-Flag expression plasmid in the presence or the absence of either CK1δ-Flag (D) or CK1ϵ-Flag (E) expression vector. The β-catenin protein was pulled down with anti-β-catenin agarose beads. The expression of β-catenin, acetylated β-catenin at K49, Flag-tagged proteins and GAPDH was detected by Western blotting. (F–H) HEK293T cells were transfected with control vector or Tip60-Flag expression plasmid in the presence or absence of either CK1δ-GFP (F) or CK1ϵ-V5 (G) or CK1α-V5 (H) expression plasmids. Whole cell lysates were immunoprecipitated with anti-Flag agarose beads. The expression of serine-phosphorylated Tip60, Tip60-Flag, CK1δ-GFP, CK1ϵ-V5, CK1α-V5 and GAPDH was measured by Western blotting. The expression of serine-phosphorylated Tip60 was detected by an anti-phospho-serine antibody.