Graphical abstract
Chemical compounds studied in this article: Benzyl alcohol (PubChem CID: 244); linalool (PubChem CID: 6549); nerolidol (PubChem CID: 5284507); β-ionone (PubChem CID: 5352481); α-terpineol (PubChem CID: 17100); acetophenone (PubChem CID: 7410); (E,Z)-2,6-nonadienal (PubChem CID: 643731); hexanal (PubChem CID: 6184); dihydro-β-ionone (PubChem CID: 519382); (E,E)-2,4-nonadienal (PubChem CID: 5283339).
Keywords: Fu brick tea, Different aroma types, Key aroma compounds, Sensory attributes, Multivariate statistical analysis
Highlights
-
•
Aroma characteristics of Fu brick tea were classified into three types.
-
•
Key aroma compounds in three aroma types of Fu brick tea were identified.
-
•
Relationship between aroma compounds and aroma attributes was illuminated.
Abstract
Aroma is one of the most important sensory properties of tea. Floral-fungal aroma type, ripe-fungal aroma type and fresh-fungal aroma type were the main aroma types of Fu brick tea by QDA. A total of 112 volatile compounds were identified and quantified in tea samples by HS-SPME/GC–MS analysis. Ten voaltiles in floral-fungal aroma type, eleven voaltiles in ripe-fungal aroma type, and eighteen voaltiles in fresh-fungal aroma type were identified as key aroma compounds for the aroma characteristics formation in three aroma types of Fu brick tea. In addition, PLS analysis revealed that 3,4-dehydro-β-ionone, dihydro-β-ionone, (+)-carotol and linalool oxide Ⅱ were the key contributors to the ‘floral and fruity’ attribute, α-terpineol contributed to ‘woody’ and ‘stale’ attributes, and thirteen aroma compounds related to ‘green’ attribute. Taken together, these findings will provide new insights into the formation mechanism of different aroma characteristics in Fu brick tea.
1. Introduction
Fu brick tea is a typical post-fermented dark tea, which is mainly produced in Hunan, Shaanxi and Zhejiang provinces of China. The manufacturing process of Fu brick tea includes steaming, piling, pressing, fermentation, and drying (Li et al., 2019, Ling et al., 2010, Xu et al., 2007). Microbial fermentation was considered as the key step in the process of Fu brick tea, which helped to form its unique ‘fungal flower’ aroma and mellow taste (Ling et al., 2010). In recent years, Fu brick tea has attracted global interest owing to its special flavor and health benefits, such as anti-obesity, anti-hyperlipidemia, anti-oxidant, anti-tumor, anti-microbial, and others (Fu et al., 2011, Li et al., 2013, Mo et al., 2008, Xu et al., 2011).
Tea aroma is one of the most important sensory properties reflecting the quality of tea (Zhu et al., 2018). ‘Fungal flower’ aroma is the basic aroma characteristics of Fu brick tea, which is formed by a variety of volatile compounds with different aroma attributes mixed together (Li et al., 2020). However, the aroma characteristics of Fu brick tea vary greatly with cultivation conditions, processing, regions and storage time. Previous study indicated that the ‘fungal flower’ aroma of aged Fu brick tea was dominated with ‘stale’ attribute and was enriched by methyl hexadecanoate, 1-octanol, methyl laurate, methyl tetradecanoate, 1-heptadecanol (Huang, Wang, Zeng, & Lai, 2011). Otherwise, the ‘fungal flower’ aroma of Fu brick tea processing in Hunan province was dominated with ‘floral’, ‘woody’, ‘green’ attributes and was enriched in methyl salicylate, acetophenone, cedrol, benzyl alcohol (Li et al., 2020). The ‘fungal flower’ aroma of Fu brick tea processing in Shaanxi province was dominated with ‘roasted’ attribute and enriched in heteroxic compounds, such as 2-pentylfuran and tetramethylpyrazine. The ‘fungal flower’ aroma of Fu brick tea producing from Zhejiang province was usually dominated by the ‘green’ attribute and was riched in low grade fatty aldehydes such as hexanal (Cao et al., 2018, Zhao et al., 2017). The ‘fungal flower’ aroma of Fu brick tea processed from Sichuan province was dominated by ‘floral and fruity’ attribute and its main volatile components are methyl salicylate, geranylacetone and β-ionone (Nie et al., 2019). To sum up, it is reasonable to speculate that there are different ‘fungal flower’ aroma characteristics, which might result in different aroma types in Fu brick tea. But up to now, there was no research focus on the aroma characteristics in different aroma types of Fu brick tea.
Therefore, the aims of the present study were to (a) classify the aroma characteristics of Fu brick tea in different aroma types according to the sensory evaluation; (b) identify the key aroma compounds contributing to the formation of different aroma characteristics in Fu brick tea; (c) illuminate the relationship between the aroma compounds and sensory attributes in Fu brick tea. This study is of significant importance for providing information to enhance our understanding of the mechanisms on different aroma characteristics formation in Fu brick tea.
2. Method and material
2.1. Chemicals
The C7-C40 n-alkanes and ethyl decanoate (99.99%) were obtained from Sigma-Aldrich (St. Louis, MO). Forty two authentic standards were purchased from J&K chemical Ltd. (Beijing, China). All the chromatographic solvents were of chromatography grade and all of the chemicals were of analytical reagent grade unless otherwise stated.
2.2. Samples and sensory analysis
Sixty Fu brick tea samples were collected from the tea market all over China. Sensory analysis was approved by Hunan Agricultural University Institutional Review Board Committee (#TSF-780-2020). Sensory evaluation was performed by eight well-trained panelists from the Tea Science Department in Hunan Agricultural University (four males and four females, aged from 25 to 55 years) according to the Chinese standards “Methodology of Sensory Evaluation of Tea” (GB/T 23776-2018). Before the experiment, each panelist had to complete a 60-hours of sensory evaluation training with different aroma types of Fu brick tea in 20 days. All participants received written information about the study, and they signed informed consent to participate. Sensory quantitative description analysis (QDA) was performed according previous study (Li, Luo, Wang, Fu, & Zeng, 2019). Briefly, 3.0 g tea sample was infused with 150 mL of boiled water for 7 min in a special tea cup. Each sample was coded with a three-digit number and randomly offered to panelists after brewing. The aroma descriptors were evaluated and discussed by panelist panel. The intensity of aroma attribute was scored by panelists using a scale from 0 to 10. Each score was expressed as mean value. Each sample was evaluated three times by each panelist in different days.
2.3. Qualitative and quantitative analysis of the volatiles in Fu brick tea by HS-SPME/GC–MS analysis
The volatile profile of Fu brick tea was extracted by headspace solid-phase micro-extraction (HS-SPME) using a 65 µm polydimethylsiloxane/divinylbenzene (PDMS/DVB) fiber (Supelco, Bellefonte, PA, USA) and was analysed using a GC system (Agilent 5977B, Agilent Technologies Inc., CA, USA) equipped with a mass spectrometer (Agilent 5977A, Agilent Technologies Inc., CA, USA), according to our previous study (Li et al., 2020). Ethyl decanoate (8.64 mg/L) was used as internal standard. An Agilent HP-5MS capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) was employed for separating the volatile compounds, and helium (purity > 99.999 %) was used as carrier gas with a constant flow rate of 1 mL/min. The injector temperature was set at 250 °C with splitless model. The GC oven temperature was set at 50 °C for 2 min, then increased at a rate of 3 °C/min to 160 °C and held for 1 min; lastly, it increased to 280 °C at a rate of 15 °C/min and kept for 1 min. The mass spectrometer conditions were set as follows: ionization mode, EI; ion source temperature, 230 °C; quadrupole temperature, 150 °C; electron energy, 70 eV; full scan mode, mass scan range 35–400 atomic mass units (amu). Each sample was analyzed three times.
Volatile compounds were identified based on retention indices (RIs), authentic standards, and mass spectra matching in the standard NIST17 library. C7-C40 n-alkane mixture was employed for determination of RIs. Each standard was mix with ‘volatile-free’ tea to obtain the standard curves. The ‘volatile-free’ tea was prepared by vacuum concentration to remove volatile compounds according to a previous study (Du, Wang, Li, Xiao, Li, & Xu, 2013). For the volatile compounds without available standards, the quantitation was carried out using the standard that had the same carbon atom or a similar functional structure (Li et al., 2020).
2.4. Odor activity values (OAVs) calculation
OAV was calculated by dividing the concentration of volatile compound with its odor threshold (OT) reported by the references. Volatile compounds with OAV > 1 were considered as an aroma-active compound, which played an important role for the aroma characteristics formation of tea samples (Mao, Lu, Li, Ye, Wei, & Tong, 2018).
2.5. Statistical analysis
The data were preprocessed by mean centering and scaling prior to analysis. Principal component analysis (PCA), hierarchical cluster analysis (HCA), orthogonal partial least squares discriminant analysis (OPLS-DA) and partial least squares analysis (PLS) were performed by SIMCA-P+ (Version 14.0, Umetrics, Umea, Sweden). Analysis of variance (ANOVA) was performed by SPSS (Version 22.0, IBM, Armonk, NY, USA). All data were presented as the mean value ± SD. Significant differences between groups were declared significant at p < 0.05.
3. Results and discussion
3.1. Aroma characteristics of Fu brick tea
After sensory evaluation, 25 samples with typical flavor characteristics of Fu brick tea were selected from 60 samples and used for subsequent sensory QDA analysis. In total, 40 attributes that described the aroma characteristics were obtained (Fig. 1A). The flavor wheel was consist of 3 tiers, 1 first-tier descriptor, 10 s-tier descriptors and 40 third-tier descriptors. The third-tier descriptors were precise descriptions to Fu brick tea, such as cherry fragrance, orchid sweet, old house smell and mushroom scent. The second-tier descriptors were the summary of the third-tier descriptors, that is, the concrete aroma attributes of Fu brick tea, which were split into 10 categories, including fermented, fruity, floral, nutty, stale, woody, green, hay flavor, fishy smell and herbal. All of the second-tier descriptors have been often used to describe the characteristics of Fu brick tea in other reports (Li et al., 2019, Li et al., 2020, Lv et al., 2014, Nie et al., 2019). It is worth mentioning that the ‘fungal flower’ aroma attribute could be a complex aroma, which might be formed by a variety of volatiles with woody, floral and mint attributes mixed together (Li et al., 2020). So, this descriptor was not presented in the flavor wheel independently. After discussion, all panelists agreed to use the five attributes with higher odor intensity as useful terms for subsequent evaluation of the aroma characteristics of Fu brick tea, including ‘floral and fruity’, ‘stale’, ‘woody’, ‘green’ and ‘herbal’.
Fig. 1.
Quantitative descriptive analysis of different aroma types of Fu brick tea. (A) Flavor wheel. (B) JHX: floral-fungal aroma type. (C) SJX: ripe-fungal aroma type. (D) QJX: fresh-fungal aroma type.
Using the QDA method, 25 samples were clustered into three groups based on sensory intensity (Fig. 1B–D). The first group had strong ‘floral and fruity’ attribute, while weaked in the other attributes. The second group, with a typical ‘stale’ attribute, also was rich in ‘woody’ attribute. The third group had strong ‘green’ attribute, whereas the other attributes were moderate. These noticeable differences suggested that the samples had significantly different flavor and intensities. Generally, eight Fu brick tea samples with strong ‘floral and fruity’ attribute were classified as ‘floral-fungal’ aroma (JHX) type, eight samples with strong ‘stale’ and ‘woody’ attributes were classified as ‘ripe-fungal’ aroma (SJX) type, and nine samples with strong ‘green’ attribute were classified as ‘fresh-fungal’ aroma (QJX) type based on the sensory evaluation.
3.2. Comparison of volatile profiles in three aroma types of Fu brick tea
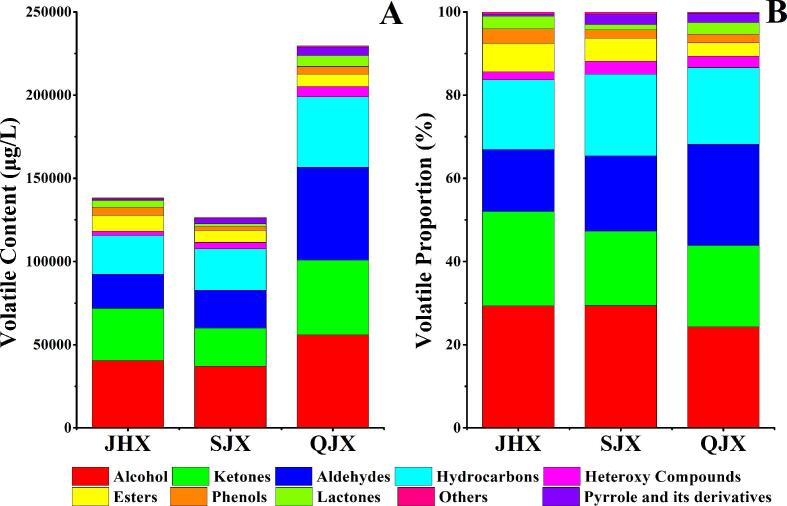
To illuminate the volatile profiles of three aroma types of Fu brick tea, a comprehensive analysis was performed by HS-SPME/GC–MS. A total of 112 volatile compounds were identified and quantified in Fu brick tea samples by absolute quantitative method (Table 1 and Table S1). For clarification, the identified compounds were further classified into 10 different sub-classes, including 26 hydrocarbons, 21 ketones, 20 alcohols, 20 aldehydes, 9 esters, 6 heteroxy compounds, 3 phenols, 2 lactones, 1 pyrrole and 4 others. Generally, the total content of volatile compounds in QJX type was much higher than that in JHX and SJX types of samples (Fig. 2A). Alcohols, ketones, aldehydes, hydrocarbons were the predominant volatile categories in Fu brick tea, which totally accounted for 85.47% in the identified volatile compounds (Fig. 2B). Previous studies also reported that alcohols, ketones, aldehydes, hydrocarbons might be the main contributing substance to the characteristic aroma formation of Fu brick tea, which was consistent with our present results (Li et al., 2019, Xu et al., 2007). Notably, alcohols were the most volatiles category in Fu brick teas (29.11%, 26.91%, and 24.32%), followed by ketones, aldehydes and hydrocarbons in the three aroma types of Fu brick tea, but it was not consistent with previous studies that reported ketones was the most abundant volatile category (Lv et al., 2014, Shi et al., 2019). This difference might be due to the absolute quantitative method used in our present study, otherwise, the relative quantitative method, such as peak area normalization method used in previous studies, might result in the lower relative content of alcohol compounds (Lv et al., 2014, Shi et al., 2019). Furthermore, alcohols, ketones and hydrocarbons were more abundant in JHX type, whereas ketones and aldehydes were more abundant in SJX and QJX types of Fu brick tea.
Table 1.
Qualitative and quantitative results of volatile components among three aroma types of Fu brick tea.
| Compounds | CAS | Quantitative ions (m/z)a | RIb/RIc | Identificationd | Concentration (μg/L) |
Proportion (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| JHX | SJX | QJX | JHX% | SJX% | QJX% | |||||
| Esters | ||||||||||
| Sebacic acid, di(2-hexyl) ester | 1000355–65-8 | 185,98,143,166 | 1851/- | MS | 1.49 ± 0.93 | 1.96 ± 1.33 | 1.46 ± 0.62 | 0.03 ± 0.01 | 0.04 ± 0.03 | 0.02 ± 0.01 |
| Octyl 4-methoxycinnamate | 5466–77-3 | 161,178,133,207 | 2329/- | MS | 9.58 ± 0.16 | 9.71 ± 0.44 | 9.69 ± 0.37 | 0.17 ± 0.03 | 0.18 ± 0.05 | 0.11 ± 0.02 |
| Diethyl phthalate | 84–66-2 | 149,177,105,121 | 1592/1602 | MS RI | 13.17 ± 4.57 | 10.81 ± 1.18 | 29.42 ± 47.91 | 0.23 ± 0.1 | 0.21 ± 0.07 | 0.35 ± 0.52 |
| Diisobutyl phthalate | 84–69-5 | 149,57,223,104 | 1870/1869 | MS RI | 26.67 ± 7.3 | 18.37 ± 7.02 | 20.76 ± 7.48 | 0.46 ± 0.16 | 0.35 ± 0.06 | 0.24 ± 0.06 |
| Methyl hexadecanoate | 112–39-0 | 74,143,87,227 | 1923/1926 | MS RI | 2.81 ± 0.23 | 2.6 ± 0.17 | 2.87 ± 0.26 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0 |
| Ethyl palmitate | 628–97-7 | 88,101,157,241 | 1990/1992 | MS RI | 0.66 ± 0.49 | 0.42 ± 0.07 | 0.54 ± 0.13 | 0.01 ± 0.01 | 0.01 ± 0 | 0.01 ± 0 |
| Dibutyl phthalate | 84–74-2 | 149,150,223,205 | 1965/1965 | MS RI | 14.09 ± 0.54 | 11.51 ± 0.59 | 12.5 ± 1.08 | 0.24 ± 0.05 | 0.22 ± 0.07 | 0.15 ± 0.03 |
| Methyl salicylate | 119–36-8 | 120,152,92,121 | 1191/1191 | MS RI STD | 294.09 ± 169.47 | 225.83 ± 219.33 | 167.88 ± 68.53 | 5.11 ± 3.06 | 4.28 ± 2.73 | 1.97 ± 0.91 |
| 2,2,4-trimethyl-1,3-pentanediol diisobutyrate | 6846–50-0 | 71,111,43,83 | 1595/- | MS | 31.62 ± 18.18 | 12.12 ± 4.83 | 26.03 ± 13.98 | 0.55 ± 0.51 | 0.23 ± 0.13 | 0.31 ± 0.14 |
| Heteroxy compounds | ||||||||||
| 1,2-dimethoxybenzene | 91–16-7 | 138,123,95,77 | 1144/1148 | MS RI STD | 37.29 ± 13.06 | 58.66 ± 32.57 | 107.37 ± 51.96 | 0.65 ± 0.28 | 1.11 ± 0.46 | 1.26 ± 0.67 |
| 1,3-dimethoxybenzene | 151–10-0 | 138,107,122,95 | 1164/- | MS | 26.7 ± 6.28 | 29.62 ± 23.61 | 30.47 ± 14.86 | 0.46 ± 0.28 | 0.56 ± 0.25 | 0.36 ± 0.17 |
| 5-methoxy-6,7-dimethylbenzofuran | 35355–35-2 | 161,131,145,176 | 1465/1468 | MS RI | 24.85 ± 5.68 | 62 ± 16.59 | 46.55 ± 14.11 | 0.43 ± 0.22 | 1.18 ± 0.43 | 0.55 ± 0.17 |
| 4-methylanisole | 104–93-8 | 122,77,91,107 | 929/- | MS STD | 6.77 ± 1.87 | 5.65 ± 1.53 | 22.61 ± 9.98 | 0.12 ± 0.02 | 0.11 ± 0.05 | 0.27 ± 0.13 |
| 2-pentylfuran | 3777–69-3 | 82,53,81,138 | 987/988 | MS RI STD | 8.73 ± 2.53 | 4.41 ± 2.87 | 20.86 ± 4.11 | 0.15 ± 0.03 | 0.08 ± 0.03 | 0.25 ± 0.05 |
| cis-anethol | 104–46-1 | 148,133,121,77 | 1281/1283 | MS RI | 5.32 ± 2.03 | 5.16 ± 1.84 | 8.28 ± 6.86 | 0.09 ± 0.03 | 0.1 ± 0.03 | 0.1 ± 0.12 |
| Ketones | ||||||||||
| Geranylacetone | 3796–70-1 | 43,69,151,107 | 1451/1449 | MS RI STD | 186.72 ± 67.15 | 145.01 ± 60.04 | 241.66 ± 54.02 | 3.24 ± 0.88 | 2.75 ± 1.74 | 2.84 ± 0.8 |
| Dihydro-β-ionone | 17283–81-7 | 126,161,176,121 | 1436/1438 | MS RI | 35.05 ± 0.44 | 35.8 ± 1.19 | 35.47 ± 0.56 | 0.61 ± 0.12 | 0.68 ± 0.22 | 0.42 ± 0.07 |
| α-ionone | 127–41-3 | 121,93,136,192 | 1425/1425 | MS RI STD | 96.18 ± 22.32 | 78.04 ± 25.69 | 120.34 ± 30.4 | 1.67 ± 0.27 | 1.48 ± 0.71 | 1.41 ± 0.39 |
| β-ionone | 79–77-6 | 177,123,135,159 | 1484/1483 | MS RI STD | 37.6 ± 12.36 | 15.59 ± 6.08 | 45.33 ± 12.72 | 0.65 ± 0.18 | 0.3 ± 0.1 | 0.53 ± 0.14 |
| 3,4-dehydro-β-ionone | 1203–08-3 | 175,91,147,190 | 1481/1482 | MS RI | 26.29 ± 7.1 | 13.89 ± 2.27 | 26.13 ± 7.49 | 0.46 ± 0.07 | 0.26 ± 0.06 | 0.31 ± 0.1 |
| Benzophenone | 119–61-9 | 105,182,77,51 | 1624/1623 | MS RI | 6.99 ± 0.94 | 7.07 ± 1.35 | 8.2 ± 4.35 | 0.12 ± 0.03 | 0.13 ± 0.05 | 0.1 ± 0.05 |
| (E)-3-nonen-2-one | 18402–83-0 | 125,55,97,140 | 1137/1134 | MS RI | 17.49 ± 5.45 | 14.67 ± 3.21 | 26.89 ± 4.53 | 0.3 ± 0.09 | 0.28 ± 0.12 | 0.32 ± 0.1 |
| 4-methyleneisophorone | 20548–00-9 | 150,135,107,91 | 1215/- | MS | 13.74 ± 0.96 | 15.37 ± 1.97 | 16.17 ± 1.17 | 0.24 ± 0.04 | 0.29 ± 0.09 | 0.19 ± 0.03 |
| 2,5-pyrrolidinedione,1-ethyl- | 2314–78-5 | 127,56,84,112 | 1132/1134 | MS RI | 14.06 ± 3.43 | 20.98 ± 17.04 | 24.37 ± 12.93 | 0.24 ± 0.04 | 0.4 ± 0.42 | 0.29 ± 0.14 |
| Dihydro-a-ionone | 31499–72-6 | 121,136,95,176 | 1413/- | MS | 79.8 ± 23.15 | 56.71 ± 33.74 | 121.26 ± 30.26 | 1.39 ± 0.37 | 1.08 ± 0.88 | 1.43 ± 0.38 |
| Hexahydrofarnesylacetone | 502–69-2 | 58,109,194,137 | 1845/1846 | MS RI | 62.23 ± 4.09 | 56.38 ± 2.32 | 64.92 ± 3.13 | 1.08 ± 0.16 | 1.07 ± 0.36 | 0.76 ± 0.16 |
| 3,5-dihydroxyacetophenone | 51863–60-6 | 152,137,109,81 | 1152/- | MS | 464.33 ± 146.01 | 133.35 ± 64.38 | 393.76 ± 131.95 | 8.07 ± 1.73 | 2.53 ± 0.74 | 4.63 ± 1.82 |
| 3-formyl-5,5-dimethyl-2-cycolohexen-1-one | 56621–35-3 | 96,152,109,68 | 1140/- | MS | 11.25 ± 4.58 | 7.9 ± 4.5 | 11.6 ± 2.73 | 0.2 ± 0.07 | 0.15 ± 0.14 | 0.14 ± 0.05 |
| Ionone | 8013–90-9 | 136,192,177,121 | 1274/- | MS | 24.78 ± 2.24 | 21.03 ± 1.82 | 26.75 ± 2.72 | 0.43 ± 0.1 | 0.4 ± 0.14 | 0.31 ± 0.07 |
| 4-t-butylpropiophone | 81561–77-5 | 147,175,190,105 | 1379/- | MS | 12.79 ± 2.06 | 10.73 ± 0.54 | 12.8 ± 1.81 | 0.22 ± 0.05 | 0.2 ± 0.06 | 0.15 ± 0.02 |
| Pulegone | 89–82-7 | 110,152,81,95 | 1244/1237 | MS RI STD | 81.33 ± 35.64 | 126.8 ± 40.55 | 149.09 ± 45.32 | 1.41 ± 0.61 | 2.41 ± 1.29 | 1.75 ± 0.72 |
| 2,2,6-trimethyl-cyclohexanone | 2408–37-9 | 82,69,140,56 | 1030/1030 | MS RI | 27.27 ± 6.13 | 7.47 ± 4.21 | 25.48 ± 7.67 | 0.47 ± 0.12 | 0.14 ± 0.05 | 0.3 ± 0.06 |
| (E,E)-3,5-octadien-2-one | 30086–02-3 | 95,81,109,43 | 1068/1074 | MS RI | 44.83 ± 19.41 | 65.01 ± 35.37 | 201.01 ± 67.52 | 0.78 ± 0.33 | 1.23 ± 0.71 | 2.36 ± 0.73 |
| Methylheptenone | 110–93-0 | 108,93,69,83 | 982/981 | MS RI STD | 24.5 ± 7.36 | 30.11 ± 10.62 | 51.9 ± 10.24 | 0.43 ± 0.13 | 0.57 ± 0.32 | 0.61 ± 0.21 |
| Acetophenone | 98–86-2 | 105,120,77,51 | 1061/1061 | MS RI STD | 37.65 ± 6.71 | 83.13 ± 79.03 | 58.11 ± 10.66 | 0.65 ± 0.16 | 1.58 ± 2.44 | 0.68 ± 0.11 |
| 3-octen-2-one | 1669–44-9 | 55,111,126,97 | 1035/1040 | MS RI STD | 0 ± 0 | 0 ± 0 | 3.02 ± 2.09 | 0 ± 0 | 0 ± 0 | 0.04 ± 0.03 |
| Hydrocarbons | ||||||||||
| Dehydro-ar-ionene | 30364–38-6 | 157,172,142,109 | 1350/1349 | MS RI | 77.44 ± 15.89 | 32.02 ± 13.1 | 59.79 ± 19.76 | 1.35 ± 0.26 | 0.61 ± 0.17 | 0.7 ± 0.2 |
| α-ionene | 475–03-6 | 159,174,160,131 | 1353/1352 | MS RI | 39.59 ± 7.15 | 21.85 ± 9.08 | 33.77 ± 9.44 | 0.69 ± 0.13 | 0.41 ± 0.06 | 0.4 ± 0.11 |
| 1,2,3-trimethoxybenzene | 634–36-6 | 168,153,177,105 | 1311/1316 | MS RI STD | 36.37 ± 10.22 | 136.91 ± 90.13 | 187.02 ± 68.07 | 0.63 ± 0.27 | 2.6 ± 1.6 | 2.2 ± 0.75 |
| 1-methylnaphthalene | 90–12-0 | 142,166,141,115 | 1304/1306 | MS RI STD | 3.28 ± 0.95 | 1.06 ± 0.98 | 5.71 ± 2.01 | 0.06 ± 0.02 | 0.02 ± 0.02 | 0.07 ± 0.03 |
| Naphthalene | 91–20-3 | 128,64,77,102 | 1178/1178 | MS RI STD | 4.68 ± 2.8 | 1.67 ± 1.99 | 7.72 ± 2.52 | 0.08 ± 0.07 | 0.03 ± 0.03 | 0.09 ± 0.03 |
| Phenylethylene | 100–42-5 | 104,78,103,58 | 883/888 | MS RI | 29.96 ± 9.59 | 32.67 ± 11.56 | 50.62 ± 22.12 | 0.52 ± 0.2 | 0.62 ± 0.28 | 0.6 ± 0.18 |
| 1-ethylcyclohexene | 1453–24-3 | 67,81,95,110 | 1008/- | MS | 62.25 ± 37.55 | 123.2 ± 57.79 | 319.75 ± 128.28 | 1.08 ± 0.59 | 2.34 ± 1.44 | 3.76 ± 1.16 |
| 2,6,10,14-tetramethylpentadecane | 1921–70-6 | 71,57,113,85 | 1703/1703 | MS RI | 8.28 ± 2.6 | 4.6 ± 1 | 6.97 ± 2.94 | 0.14 ± 0.08 | 0.09 ± 0.04 | 0.08 ± 0.03 |
| 2,2′,5,5′-tetramethylbiphenyl | 3075–84-1 | 195,210,165,180 | 1680/1675 | MS RI | 5.95 ± 2.18 | 4.56 ± 0.56 | 6.04 ± 1.44 | 0.1 ± 0.05 | 0.09 ± 0.03 | 0.07 ± 0.03 |
| α-cedrene | 469–61-4 | 119,161,204,93 | 1409/1409 | MS RI STD | 139.09 ± 128.7 | 244.08 ± 231.94 | 125.48 ± 55.02 | 2.42 ± 2.15 | 4.63 ± 4.44 | 1.48 ± 0.52 |
| Calamenene | 483–77-2 | 159,204,137,123 | 1521/1522 | MS RI | 33.15 ± 14.82 | 31.97 ± 18.2 | 33.85 ± 8.1 | 0.58 ± 0.26 | 0.61 ± 0.16 | 0.4 ± 0.07 |
| 3,4-dimethoxytoluene | 494–99-5 | 152,137,167,109 | 1236/1241 | MS RI STD | 11.9 ± 2.51 | 10.15 ± 8.01 | 20.11 ± 14.7 | 0.21 ± 0.21 | 0.19 ± 0.1 | 0.24 ± 0.17 |
| 3,5-dihydroxyamylbenzene | 500–66-3 | 124,180,137,81 | 1523/1528 | MS RI | 111.34 ± 30.54 | 89.39 ± 36.43 | 162.6 ± 37.48 | 1.93 ± 0.44 | 1.7 ± 0.67 | 1.91 ± 0.42 |
| (+)-β-cedrene | 546–28-1 | 161,204,120,93 | 1418/1420 | MS RI | 25.43 ± 13.95 | 37.82 ± 26.96 | 25.57 ± 6.71 | 0.44 ± 0.26 | 0.72 ± 0.51 | 0.3 ± 0.06 |
| 1,6-dimethylnaphthalene | 575–43-9 | 156,141,177,121 | 1412/1410 | MS RI | 23.73 ± 4.56 | 17.24 ± 4.2 | 29.21 ± 4.88 | 0.41 ± 0.09 | 0.33 ± 0.11 | 0.34 ± 0.07 |
| 3,4-diethylbiphenyl | 61141–66-0 | 195,165,210,180 | 1708/- | MS | 5.13 ± 1.25 | 4.11 ± 0.35 | 5.11 ± 1.04 | 0.09 ± 0.03 | 0.08 ± 0.03 | 0.06 ± 0.02 |
| Hexadecane | 638–36-8 | 71,57,43,85 | 1806/1809 | MS RI | 5.12 ± 1.43 | 3.27 ± 0.39 | 4.29 ± 1.14 | 0.09 ± 0.04 | 0.06 ± 0.02 | 0.05 ± 0.01 |
| 2,6-di-tert-butyl-p-benzoquinone | 719–22-2 | 135,220,177,121 | 1464/- | MS | 30.61 ± 12.98 | 24.49 ± 7.52 | 34.02 ± 19.35 | 0.53 ± 0.2 | 0.46 ± 0.18 | 0.4 ± 0.25 |
| 2-vinylnaphthalene | 827–54-3 | 154,153,128,76 | 1374/1381 | MS RI | 28.88 ± 7.07 | 20.29 ± 5.3 | 34.13 ± 7.65 | 0.5 ± 0.09 | 0.38 ± 0.08 | 0.4 ± 0.1 |
| Fluorene | 86–73-7 | 166,165,83,109 | 1573/1572 | MS RI | 71.62 ± 40.97 | 35.29 ± 37.67 | 94.06 ± 64.19 | 1.24 ± 0.67 | 0.67 ± 0.44 | 1.11 ± 1.09 |
| Heptadecane | 629–78-7 | 57,71,85,43 | 1697/1700 | MS RI | 12.4 ± 0.36 | 11.8 ± 0.09 | 12.06 ± 0.19 | 0.22 ± 0.05 | 0.22 ± 0.07 | 0.14 ± 0.02 |
| m-xylene | 108–38-3 | 91,105,77,56 | 860/864 | MS RI | 150.04 ± 41.27 | 116.47 ± 68.89 | 267.14 ± 106.14 | 2.61 ± 0.63 | 2.21 ± 0.97 | 3.14 ± 0.91 |
| Phenanthrene | 85–01-8 | 178,176,111,151 | 1775/1778 | MS RI | 4.96 ± 0.47 | 4.64 ± 0.7 | 5.53 ± 0.76 | 0.09 ± 0.01 | 0.09 ± 0.02 | 0.07 ± 0.01 |
| Acenaphthene | 83–32-9 | 153,77,154,123 | 1476/- | MS | 26.92 ± 14.2 | 13.16 ± 3.39 | 14.97 ± 2.27 | 0.47 ± 0.22 | 0.25 ± 0.05 | 0.18 ± 0.04 |
| Octadecane | 593–45-3 | 57,71,85,99 | 1796/1800 | MS RI | 11.9 ± 0.14 | 11.63 ± 0.04 | 11.72 ± 0.06 | 0.21 ± 0.04 | 0.22 ± 0.07 | 0.14 ± 0.02 |
| 2-methylnaphthalene | 91–57-6 | 142,141,115,139 | 1286/1285 | MS RI STD | 4.35 ± 1.45 | 1.22 ± 1.36 | 8.81 ± 3.37 | 0.08 ± 0.03 | 0.02 ± 0.02 | 0.1 ± 0.05 |
| Aldehydes | ||||||||||
| trans-2-hexenal | 6728–26-3 | 69,83,98,55 | 843/846 | MS RI STD | 321.75 ± 87.14 | 308.27 ± 86.85 | 565.18 ± 237.91 | 5.59 ± 1.2 | 5.85 ± 2.11 | 6.64 ± 2.07 |
| Hexanal | 66–25-1 | 56,82,95,100 | 788/800 | MS RI STD | 129.62 ± 96.94 | 233.01 ± 88.35 | 572.45 ± 256.08 | 2.25 ± 1.8 | 4.42 ± 2.38 | 6.73 ± 2.35 |
| Benzaldehyde | 100–52-7 | 106,51,78,106 | 954/957 | MS RI STD | 62.64 ± 8.63 | 57.39 ± 17.62 | 123.65 ± 31.86 | 1.09 ± 0.11 | 1.09 ± 0.22 | 1.45 ± 0.3 |
| Citral | 5392–40-5 | 69,84,97,137 | 1266/1268 | MS RI | 8.74 ± 1.83 | 19.73 ± 11.09 | 17.72 ± 6.94 | 0.15 ± 0.02 | 0.37 ± 0.14 | 0.21 ± 0.09 |
| (E)-2-octenal | 2548–87-0 | 70,55,41,83 | 1054/1056 | MS RI | 31.09 ± 9.04 | 39.92 ± 10.16 | 77.26 ± 28.24 | 0.54 ± 0.18 | 0.76 ± 0.36 | 0.91 ± 0.28 |
| Octanal | 124–13-0 | 43,84,100,110 | 1000/1001 | MS RI | 20.24 ± 14.75 | 17.4 ± 17.68 | 12.83 ± 18.77 | 0.35 ± 0.28 | 0.33 ± 0.42 | 0.15 ± 0.24 |
| Nonanal | 124–19-6 | 57,98,82,114 | 1101/1101 | MS RI | 56.71 ± 36.96 | 39.64 ± 24.15 | 177.34 ± 85.64 | 0.99 ± 0.67 | 0.75 ± 0.57 | 2.08 ± 0.78 |
| trans-2-decenal | 3913–81-3 | 70,83,121,55 | 1257/1258 | MS RI | 15.23 ± 0.19 | 16.74 ± 2.9 | 16.83 ± 0.95 | 0.26 ± 0.05 | 0.32 ± 0.09 | 0.2 ± 0.04 |
| (E,E)-2,4-heptadienal | 4313–03-5 | 81,67,79,110 | 993/997 | MS RI STD | 23.04 ± 17.14 | 37.17 ± 22.92 | 89.97 ± 62.12 | 0.4 ± 0.25 | 0.71 ± 0.56 | 1.06 ± 0.65 |
| (E,E)-2,4-decadienal | 25152–84-5 | 81,95,152,67 | 1288/- | MS | 2.95 ± 0.04 | 2.97 ± 0.08 | 3.37 ± 0.34 | 0.05 ± 0.01 | 0.06 ± 0.02 | 0.04 ± 0.01 |
| (E,Z)-2,6-nonadienal | 557–48-2 | 41,70,94,69 | 1150/1155 | MS RI | 66.22 ± 3.83 | 64.37 ± 5.24 | 86.92 ± 14.74 | 1.15 ± 0.21 | 1.22 ± 0.31 | 1.02 ± 0.07 |
| (E,E)-2,4-nonadienal | 5910–87-2 | 81,138,95,67 | 1210/1213 | MS RI STD | 8.92 ± 10.36 | 26.47 ± 22.54 | 123.15 ± 68.78 | 0.15 ± 0.17 | 0.5 ± 0.59 | 1.45 ± 0.79 |
| Decanal | 112–31-2 | 57,82,95,112 | 1203/1200 | MS RI STD | 1.46 ± 2.42 | 0 ± 0 | 11.12 ± 9.05 | 0.03 ± 0.04 | 0 ± 0 | 0.13 ± 0.09 |
| Heptaldehyde | 111–71-7 | 70,81,55,96 | 896/899 | MS RI STD | 22.93 ± 8.56 | 21.24 ± 6.64 | 59.13 ± 22.31 | 0.4 ± 0.14 | 0.4 ± 0.13 | 0.7 ± 0.2 |
| β-cyclocitral | 432–25-7 | 152,137,123,109 | 1217/1218 | MS RI STD | 29.31 ± 4.65 | 21.27 ± 6.36 | 40.08 ± 8.4 | 0.51 ± 0.07 | 0.4 ± 0.11 | 0.47 ± 0.1 |
| 2,3-dihydro-2,2,6-trimethylbenzalhyde | 116–26-7 | 107,121,150,91 | 1196/1196 | MS RI | 19.78 ± 3.66 | 10.16 ± 1.26 | 18.56 ± 2.74 | 0.34 ± 0.05 | 0.19 ± 0.05 | 0.22 ± 0.05 |
| 2-undecenal | 2463–77-6 | 70,83,97,121 | 1361/1362 | MS RI | 15.12 ± 0.08 | 15.16 ± 0.11 | 15.54 ± 0.22 | 0.26 ± 0.05 | 0.29 ± 0.09 | 0.18 ± 0.03 |
| 1-cyclohexene-1-acetaldehyde | 472–67-2 | 104,107,151,123 | 1253/1254 | MS RI | 7.04 ± 0.32 | 7.18 ± 0.92 | 9.71 ± 3.05 | 0.12 ± 0.02 | 0.14 ± 0.05 | 0.11 ± 0.05 |
| trans-2-heptenal | 18829–55-5 | 83,55,83,70 | 950/956 | MS RI | 5.47 ± 2.81 | 11.51 ± 5.54 | 25.73 ± 10.37 | 0.1 ± 0.05 | 0.22 ± 0.17 | 0.3 ± 0.11 |
| Benzylcarboxaldehyde | 122–78-1 | 91,120,92,65 | 1039/1040 | MS RI STD | 9.87 ± 18.83 | 3.34 ± 5.96 | 20.5 ± 10.23 | 0.17 ± 0.26 | 0.06 ± 0.08 | 0.24 ± 0.12 |
| Phenols | ||||||||||
| 4-amino-3-methylphenol | 2835–99-6 | 123,122,106,94 | 1045/- | MS | 164.56 ± 50.11 | 66.69 ± 14.1 | 132.95 ± 47.7 | 2.86 ± 0.93 | 1.26 ± 0.42 | 1.56 ± 0.71 |
| 4-(2-butyl)phenol | 99–71-8 | 121,150,93,109 | 1279/- | MS | 38.22 ± 2.53 | 37 ± 0.95 | 42.69 ± 3.75 | 0.66 ± 0.13 | 0.7 ± 0.22 | 0.5 ± 0.13 |
| 2,4-di-tert-butylphenol | 96–76-4 | 191,57,162,206 | 1509/1514 | MS RI STD | 0.19 ± 0.44 | 0.11 ± 0.28 | 0.26 ± 0.63 | 0 ± 0.01 | 0 ± 0.01 | 0 ± 0.01 |
| Alcohols | ||||||||||
| α-terpineol | 98–55-5 | 121,136,93,81 | 1188/1188 | MS RI STD | 68.35 ± 19.5 | 254.28 ± 276.39 | 48.79 ± 60.26 | 1.19 ± 0.34 | 4.82 ± 3.59 | 0.57 ± 0.81 |
| Geraniol | 106–24-1 | 69,123,93,111 | 1251/1251 | MS RI STD | 40.92 ± 30.75 | 128.08 ± 251.68 | 39.99 ± 14.37 | 0.71 ± 0.43 | 2.43 ± 3.28 | 0.47 ± 0.13 |
| 1-octen-3-ol | 3391–86-4 | 57,72,85,99 | 974/974 | MS RI STD | 27.41 ± 4.44 | 23.56 ± 3.97 | 44.71 ± 5.07 | 0.48 ± 0.05 | 0.45 ± 0.15 | 0.53 ± 0.11 |
| Cedrol | 77–53-2 | 95,150,135,119 | 1598/1598 | MS RI STD | 64.44 ± 55.53 | 106.33 ± 113.46 | 84.06 ± 43.35 | 1.12 ± 1.07 | 2.02 ± 2.27 | 0.99 ± 0.5 |
| Linalool | 78–70-6 | 93,121,71,136 | 1098/1098 | MS RI STD | 140.67 ± 74.52 | 109.3 ± 134.63 | 97.9 ± 55.73 | 2.44 ± 1.46 | 2.07 ± 1.89 | 1.15 ± 0.67 |
| Phenethyl alcohol | 60–12-8 | 91,122,92,65 | 1109/1108 | MS RI STD | 232.35 ± 66.59 | 230.19 ± 156.58 | 446.62 ± 208.42 | 4.04 ± 1.03 | 4.37 ± 1.97 | 5.25 ± 2.72 |
| cis-1-p-menthanol | 3901–95-9 | 71,98,123,141 | 1128/- | MS | 5.04 ± 1.85 | 4.09 ± 0.86 | 7.26 ± 2.76 | 0.09 ± 0.05 | 0.08 ± 0.04 | 0.09 ± 0.05 |
| (-)-α-cadinol | 481–34-5 | 95,161,105,204 | 1656/1652 | MS RI | 0.73 ± 1.03 | 0.16 ± 0.26 | 0.54 ± 0.79 | 0.01 ± 0.02 | 0 ± 0 | 0.01 ± 0.01 |
| (R,R)-(+)-hydrobenzoin | 52340–78-0 | 107,108,79,77 | 1020/- | MS | 20.57 ± 1.57 | 19.39 ± 6.11 | 18.4 ± 2.3 | 0.36 ± 0.06 | 0.37 ± 0.16 | 0.22 ± 0.04 |
| 2,6-dimethylcyclohexanol | 5337–72-4 | 71,95,110,128 | 1105/- | MS | 57.69 ± 13.78 | 38 ± 11.06 | 77.47 ± 12.79 | 1 ± 0.18 | 0.72 ± 0.31 | 0.91 ± 0.16 |
| Ledol | 577–27-5 | 58,107,182,95 | 1402/- | MS | 12.39 ± 0.57 | 11.97 ± 0.27 | 12.79 ± 0.46 | 0.22 ± 0.04 | 0.23 ± 0.07 | 0.15 ± 0.03 |
| t-cadinol | 5937–11-1 | 161,95,204,121 | 1642/1647 | MS RI | 11.37 ± 1.9 | 9.95 ± 0.54 | 10.3 ± 0.39 | 0.2 ± 0.06 | 0.19 ± 0.05 | 0.12 ± 0.02 |
| Linalool oxide (trans-pyranoid) | 39028–58-5 | 68,94,151,59 | 1166/- | MS | 62.29 ± 35.63 | 50.02 ± 22.94 | 47.01 ± 18.87 | 1.08 ± 0.45 | 0.95 ± 0.21 | 0.55 ± 0.16 |
| Terpinen-4-ol | 562–74-3 | 71,93,111,154 | 1174/1179 | MS RI STD | 17.16 ± 4.83 | 9.47 ± 3.13 | 10.7 ± 4.72 | 0.3 ± 0.08 | 0.18 ± 0.04 | 0.13 ± 0.06 |
| 2-ethylhexanol | 104–76-7 | 57,98,112,83 | 1025/1026 | MS RI | 184.45 ± 148.43 | 196.17 ± 131.88 | 456.38 ± 387.04 | 3.2 ± 2.85 | 3.72 ± 3.23 | 5.37 ± 4.08 |
| Benzyl alcohol | 100–51-6 | 108,79,91,107 | 1029/1031 | MS RI STD | 522.72 ± 307.55 | 215.1 ± 180.57 | 517.89 ± 219.95 | 9.08 ± 4.09 | 4.08 ± 2.81 | 6.09 ± 2.14 |
| Linalool oxide Ⅰ | 5989–33-3 | 59,94,111,68 | 1070/1068 | MS RI | 49.73 ± 12.63 | 46.54 ± 17.17 | 37.56 ± 7.71 | 0.86 ± 0.2 | 0.88 ± 0.09 | 0.44 ± 0.13 |
| Linalool oxide Ⅱ | 34995–77-2 | 59,111,137,94 | 1086/1085 | MS RI | 79.4 ± 35.27 | 67.39 ± 41.48 | 55.33 ± 15.11 | 1.38 ± 0.58 | 1.28 ± 0.41 | 0.65 ± 0.17 |
| Nerolidol | 7212–44-4 | 69,81,93,107 | 1561/1569 | MS RI STD | 72.32 ± 98.35 | 7.9 ± 15.08 | 28.79 ± 21.15 | 1.26 ± 1.39 | 0.15 ± 0.32 | 0.34 ± 0.25 |
| Heptanol | 111–70-6 | 70,56,69,98 | 964/969 | MS RI STD | 20.01 ± 2.36 | 21.3 ± 2.03 | 27.08 ± 4.43 | 0.35 ± 0.05 | 0.4 ± 0.13 | 0.32 ± 0.11 |
| Pyrrole | ||||||||||
| Tetramethylpyrazine | 1124–11-4 | 136,54,42,95 | 1083/- | MS STD | 31.66 ± 2.51 | 131.83 ± 166.84 | 186.31 ± 148.7 | 0.55 ± 0.08 | 2.5 ± 2.06 | 2.19 ± 1.34 |
| Lactones | ||||||||||
| Dihydroactinolide | 17092–92-1 | 111,137,180,109 | 1525/1526 | MS RI STD | 81.5 ± 82.12 | 7.03 ± 13.12 | 128.46 ± 56.33 | 1.42 ± 1.08 | 0.13 ± 0.17 | 1.51 ± 0.59 |
| γ-nonanolactone | 104–61-0 | 85,119,162,91 | 1359/1360 | MS RI | 90.57 ± 43.11 | 60.18 ± 25.26 | 111.24 ± 64.52 | 1.57 ± 0.57 | 1.14 ± 0.79 | 1.31 ± 1.17 |
| Others | ||||||||||
| Caffeine | 58–08-2 | 194,109,85,71 | 1846/1846 | MS RI | 1.12 ± 0.41 | 1.16 ± 0.47 | 1.38 ± 0.31 | 0.02 ± 0 | 0.02 ± 0.01 | 0.02 ± 0 |
| Oleic acid | 112–80-1 | 57,69,95,71 | 2172/2140 | MS RI | 11.54 ± 0 | 11.53 ± 0 | 11.54 ± 0 | 0.2 ± 0.04 | 0.22 ± 0.07 | 0.14 ± 0.02 |
| Palmitic acid | 57–10-3 | 73,129,57,83 | 1958/1963 | MS RI | 6.98 ± 2.34 | 5.33 ± 1.68 | 7.57 ± 2.03 | 0.12 ± 0.02 | 0.1 ± 0.03 | 0.09 ± 0.02 |
| (+)-carotol | 465–28-1 | 161,119,71,85 | 1768/- | MS | 9.32 ± 0.22 | 9.46 ± 0.41 | 9.45 ± 0.23 | 0.16 ± 0.04 | 0.18 ± 0.05 | 0.11 ± 0.02 |
Ions monitored for quantitation. The underlined ions were the quantified ones while the others were the identified ones.
Retention index of compounds on HP-5MS.
Retention index of compounds in reference.
“MS” mass spetrum comparison using NIST17 library. “RI” retention index in agreement with literature value. “STD” confirmed by authenic standards.
Fig. 2.
Differences of the volatiles content (A) and proportion (B) in three aroma types of Fu brick tea. JHX: floral-fungal aroma type. SJX: ripe-fungal aroma type. QJX: fresh-fungal aroma type.
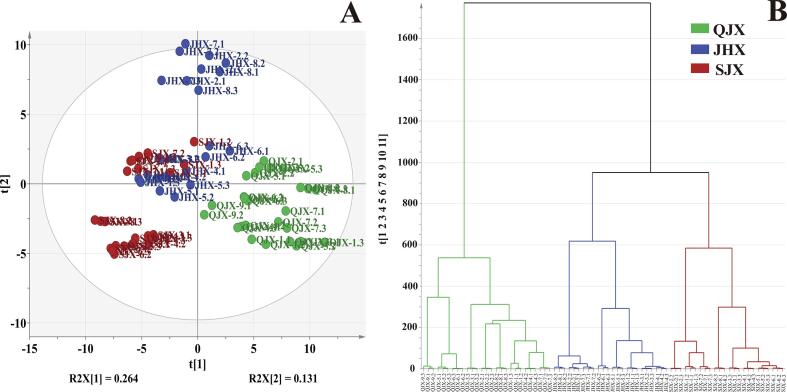
To obtain a preliminary overview of similarities and differences among the different aroma types of Fu brick tea, unsupervised PCA were carried out based on the absolute content of identified volatile compounds, and most of the chemical phenotypes of the samples were well discriminated according to the aroma types of Fu brick tea. As shown in Fig. 3A (PC1, 26.4%; PC2, 13.1%; R2X = 0.852), there was a clear differentiation among the three aroma types of Fu brick tea samples, suggesting that the volatile profile of tea samples differed dramatically in their content levels. Furthermore, HCA analysis was also performed to distinguish the similarities and differences of volatile composition and content in different tea samples, which was consistent with the PCA results (Fig. 3B). Overall, Fu brick teas could be divided into three groups based on their aroma types: JHX type (JHX-1 ∼ JHX-8), SJX type (SJX-1 ∼ SJX-8) and QJX type (QJX-1 ∼ QJX-9). However, the absolute content of volatile compounds could not reflect the contribution of each compounds for the aroma characteristics formation in tea samples due to the absolute content of volatiles in QJX type samples was much higher than that in the other two types. Therefore, the percentage content of each volatile was used to explore the different volatile components in three types of Fu brick tea. Based on these results, three OPLS-DA models were established to investigate the differential volatiles contributing to distinguish different aroma types, including OPLS-DA model Ⅰ (JHX type and SJX type), OPLS-DA models Ⅱ (JHX type and QJX type) and OPLS-DA models III (SJX type and QJX type), respectively (Fig. S1). Two conditions need to be fulfilled for identifying the discriminatory volatiles in three aroma types Fu brick tea: the values of predictive component variable importance in the projection (VIP) ≥ 1 and p-value ≤ 0.05. Based on these criteria, 75 volatiles were identified as discriminatory volatile compounds (VIP > 1.0, p < 0.05) among the three groups. Obviously, the level of these discriminatory volatiles showed a dramatically different among the three aroma types of Fu brick tea (Fig. S2). In total, there were 29 discriminatory volatile compounds displayed the highest amount in JHX type samples, while 27 and 19 discriminatory volatile compounds had the highest content in SJX type and QJX type samples, respectively. Moreover, the higher level of discriminatory ketone and alcohol compounds in JHX type of Fu brick tea, including β-ionone, 3,5-dihydroxyacetophenone, ionone, benzyl alcohol, linalool, nerolidol, linalool oxide (trans-pyranoid), might cause the aroma characteristics of JHX type of Fu brick tea differentiate from that in the other two aroma types (Huang et al., 2011, Lv et al., 2014, Shi et al., 2019). Also, the higher level of discriminatory ketone and aldehyde compounds in SJX type of Fu brick tea, such as acetophenone, dihydro-β-ionone, pulegone, (E,Z)-2,6-nonadienal, might contributed to the difference formation in the aroma characteristics of SJX type of Fu brick tea, as well as the higher level of discriminatory aldehyde compounds in QJX type of Fu brick tea, including hexanal, (E,E)-2,4-heptadienal, benzaldehyde, nonanal, (E,E)-2,4-nonadienal (Li et al., 2019, Li et al., 2020, Nie et al., 2019, Shi et al., 2019, Xu et al., 2007).
Fig. 3.
PCA (A) and HCA (B) analysis based on the identified volatile compounds in different aroma types of Fu brick tea. JHX: floral-fungal aroma type. SJX: ripe-fungal aroma type. QJX: fresh-fungal aroma type.
3.3. Differential aroma compounds in three aroma types of Fu brick tea
It is well known that the contribution of a volatile compound to the overall aroma of tea was not only depended on the concentration but also relied on its odor threshold. In our study, the odor descriptions and OAV values of volatile compounds were presented in Table S2, based on the reported references. Generally, there were 54 volatiles with OAV > 1 and 17 volatiles with OAV < 1. It has shown that the OAV value was directly proportional to the contribution degree of aroma (Liu, Zhou, & Xu, 2008). In this study, there are 28 aroma compounds with OAV > 10 were common to the three aroma types of Fu brick tea, including eight aroma compounds with floral attribute, nine with green attribute, four with woody attribute, two with fatty attribute, one with pungent attribute, two with stale attribute, one with earthy attribute and one with fruity attribute. All these aroma compounds provided important contribution to the basic aroma characteristics formation of Fu brick tea. Xu et al. (2007) reported that the compounds with floral and stale attributes in combination with some components of the raw material, which were together contributed to the ‘fungal flower’ aroma characteristics formation in Fu brick tea. However, some volatile compounds lacking OAV values might also contribute to the aroma characteristics formation of Fu brick tea. Fox example, (+)-β-cedrene and α-cedrene were found to be important compounds contributing to the woody flavour in Pu-erh tea (Xu et al., 2016, Lv et al., 2012). t-Cadinol was considered to have an important contribution to the formation of on the chestnut-like aroma in green tea (Wang, Hua, Jiang, Yang, Wang, & Yuan, 2020).
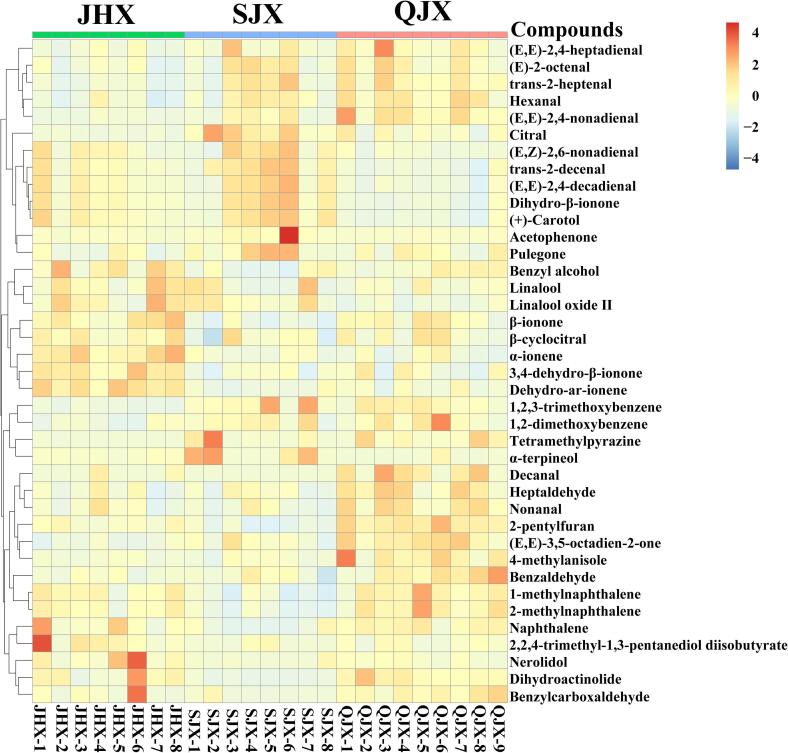
Combined with OPLS-DA results, 39 aroma compounds were considered as the discriminatory aroma compounds (VIP > 1, p < 0.05, OAV > 1) among the three aroma types of Fu brick tea (Fig. 4). For JHX type of Fu brick tea, there are 10 aroma compounds considered as discriminatory aroma compounds, including benzyl alcohol, linalool, linalool oxide Ⅱ dehydro-ar-ionene, nerolidol, α-ionene, β-ionone, 2,2,4-trimethyl-1,3-pentanediol diisobutyrate, β-cyclocitral, 3,4-dehydro-β-ionone. The above discriminatory aroma compounds were presented at the highest level in the JHX type of Fu brick tea when compared with the other two aroma types. The aroma characteristics of JHX type of Fu brick tea was dominated by floral and fruity attributes, mixing with stale and woody attributes to form the floral-fungal aroma characteristics. Benzyl alcohol has the highest content in all volatile components of Fu brick tea, accounting for 6.4%. Also, previous studies reported that benzyl alcohol provided an important contribution to the floral attribute in Fu brick tea (Li et al., 2020, Wang et al., 2019). Linalool and linalool oxide Ⅱ possessing typical floral attribute, could enhance the intense of floral attribute and decreased the intense of roasted attribute in instant white tea (Demyttenaere & Willemen, 1998). In addition, those two compounds were also identified as the important contributors to the ‘fungal flower’ aroma formation of Fu brick tea (Xu et al., 2007). Nerolidol is a sesquiterpene present in many teas, which was one of the most important factors to form the floral and fruity aroma in oolong tea and was also identified as the key contributor to the roses and apples aroma of black tea (Xu et al., 2016, Zhu et al., 2015). β-Ionone with floral attribute was derived from carotenoids, which played an important role in the aroma characteristic formation of many teas, such as Fu brick tea, due to its extremely low odor threshold (Li et al., 2020). Furthermore, β-cyclocitral, impart fruity attribute, which could be produced by the degradation of β-carotene, was also considered as one of the important compounds to form the ‘fungal flower’ aroma of Fu brick tea (Wang, Ma, Shi, Zhu, Lin, & Lv, 2020). Dehydro-ar-ionene presented with woody attribute, which was generated by the hydrolysis of aroma glycosides and contributed to the aroma characteristics formation in oolong tea (Guo, Ho, Wan, Zhu, Liu, & Wen, 2021).
Fig. 4.
Heatmap analysis for the discriminatory aroma compounds among the three aroma types of Fu brick tea. JHX: floral-fungal aroma type. SJX: ripe-fungal aroma type. QJX: fresh-fungal aroma type.
For the SJX type of Fu brick tea, 11 aroma compounds presented the highest level compared with the other two aroma types were identified as discriminatory aroma compounds, including tetramethylpyrazine, α-terpineol, 1,2,3-trimethoxybenzene, pulegone, acetophenone, (E,Z)-2,6-nonadienal, dihydro-β-ionone, citral, trans-2-decenal, (+)-carotol, (E,E)-2,4-decadienal. Those compounds impart woody, stale and fatty attributes were found to dominate in SJX type of Fu brick tea, combining with those of which contributed to roasted, floral, fruity, green and mint scents to form the ripe-fungal aroma characteristics. α-Terpineol with woody note has been considered as an important aroma compound for the smoky or stale aroma characteristics in Pu-erh tea, which was hydrolyzed from glycoside precursors by microbial enzyme during the post-fermentation process (Lv, Zhong, Lin, Wang, Tan, & Guo, 2012). Dehydro-β-ionone with woody note was widely presence in tea, as the fermented product of β-ionone by fungal enzyme. It was also reported to be the key aroma compound for the ‘aging fragrance’ characteristics formation in the aging-storage Qingzhuan tea (Zhang et al., 2021). 1,2,3-trimethoxybenzene is well known as a typical stale compound that was considered to be the most important contributor to the stale characteristics of Pu-erh tea (Lv et al., 2014). It was transformed by fungal methylate action from gallic acid and the methylation of phenolic hydroxyl groups by Aspergillus niger during the pile-fermentation process (Lv et al., 2014). (E,E)-2,4-decadienal with fatty note was also considered as an important aroma compound in Chinese dark tea (Cao et al., 2018). Tetramethylpyrazine is a pyrazine compound with roasted attribute, which contributed to the characteristic aging fragrance formation of Chinese liquor and aged vinegar (Zhang et al., 2020, Zhu et al., 2016). Furthermore, acetophenone was mainly contributed to a floral note, which was identified as the basic aroma compounds of Fu brick tea (Li et al., 2020, Shi et al., 2019). Citral is a monoterpene aldehyde with fruity note that could be converted from carotenoids during the post-fermentation of tea, and it was considered to be a major constituent of essential oils in many lemon-scented aromatic plants (Dudai, Weinstein, Krup, Rabinski, & Ofir, 2005). (E,Z)-2,6-nonadienal was the key odorant in Chinese black teas and Darjeeling black tea, which could enhance green aroma in tea infusion (Chen et al., 2019, Greger and Schieberle, 2007). Pulegone was identified as the main aroma compound of peppermint that was usually used as herbal teas, spices and essential oil, due to its minty note and high relative odor intensity (Diaz Maroto, Castillo, Castro Vazquez, Gonzalez Vinas, & Perez Coello, 2007).
For the QJX type of Fu brick tea, there were 18 compounds considered as discriminatory aroma compounds, including hexanal, (E,E)-3,5-octadien-2-one, nonanal, dihydroactinolide, benzaldehyde, (E,E)-2,4-nonadienal, 1,2-dimethoxybenzene, (E,E)-2,4-heptadienal, (E)-2-octenal, heptaldehyde, (E)-2-heptenal, 4-methylanisole, 2-pentylfuran, benzylcarboxaldehyde, decanal, 2-methylnaphthalene, naphthalene, 1-methylnaphthalene. The highest content of these 18 compounds were found in the fresh-fungal aroma type of Fu brick tea compared with the other types. The aroma characteristics of QJX type of Fu brick tea was mainly formed by green attribute, combining with fruity, stale and pungent notes to form the fresh-fungal aroma characteristics. (E,E)-3,5-Octadien-2-one, (E,E)-2,4-heptadienal, (E,E)-2,4-nonadienal, benzylcarboxaldehyde and decanal exhibit a freshly cut grass smell and were all identified as the key aroma compounds to form green attribute of the post-fermentation dark tea in previous studies, such as Fu brick tea and Pu-erh tea (Cao et al., 2018, Li et al., 2020, Lv et al., 2012, Shi et al., 2019). Hexanal, (E)-2-octenal, 2-pentylfuran and benzylcarboxaldehyde also with typical green attribute was often reported to contribute to form the characteristic green aroma of non-fermentation or full-fermentation tea, such as green tea and black tea (Kang et al., 2019, Wang et al., 2020, Zhu et al., 2018). Benzaldehyde is an important odor compound with fruity note, which often present in the chestnut-like aroma of green tea (Zhu et al., 2018). Naphthalene, 1-methylnaphthalene and 2-methylnaphthalene contributed with pungent and herbal-like notes, which could be degraded by microorganisms (Tanguler, Selli, Sen, Cabaroglu, & Erten, 2017). Naphthalene with pungent note, converted from long-chain hydrocarbons, was identified as the key odorant in yellow tea and Wuyi rock tea (Liu et al., 2021, Shi et al., 2021). 1-Methylnaphthalene also possessing pungent note was reported to contribute to the aroma of Liupao tea, which was usually used as a potential indicator to evaluate the degree of fermentation (Ma et al., 2020). 1,2-Dimethoxybenzene mainly contributed to a stale note, which was regard as important compound in the formation of the special flavor in Pu-erh tea (Lv et al., 2012).
3.4. Relationship between aroma compounds and sensory attributes in three aroma types of Fu brick tea
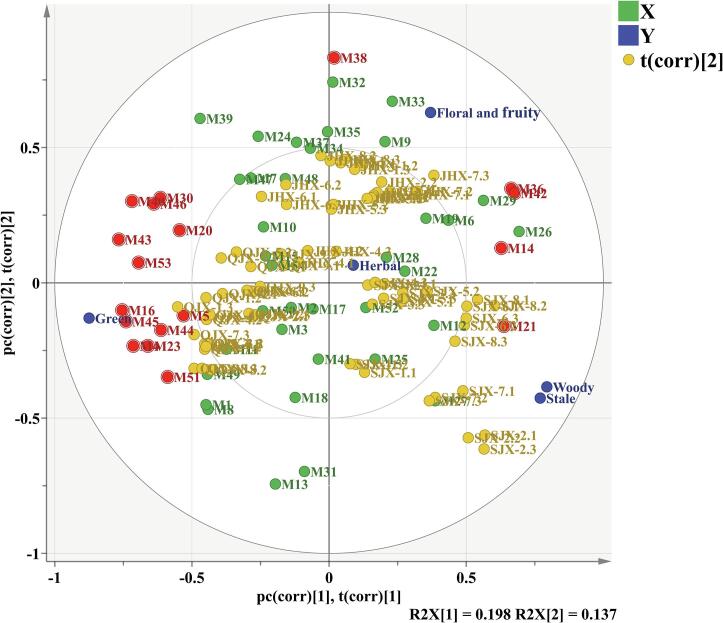
To explore the relationships among the aroma compounds (OAV > 1), sensory attributes and tea samples, PLS analysis was performed. Two latent variables were included in the PLS model, which represents 82.8% of X-matrix variance, explained 79.6% of Y-matrix variance. As shown in Fig. 5, samples appeared to be divided into three groups according to the different aroma types. Among them, floral-fungal aroma type of samples were mainly distributed at the positive value of PC1 and PC2, ripe-fungal aroma type of samples were mainly located at the positive values of PC1 and negative values of PC2, fresh-fungal aroma type of samples were mainly distributed at the negative value of PC1 and PC2. The first PC was defined by the aroma descriptors showing ‘green’ attribute on the negative dimension and ‘herbal’, ‘floral and fruity’, ‘stale’, ‘woody’ attributes on the positive dimension.
Fig. 5.
PLS plots for the tea samples, the sensory attributes and aroma compounds (OAV>1). M38: 3,4-dehydro-β-ionone. M36: dihydro-β-ionone. M42: (+)-carotal. M14: linalool oxide. M21: α-terpineol. M43: 2-pentylfuran. M45: decanal. M16: nonanal. M4: heptaldehyde. M40: dihydroactinolide. M53: 4-methylanisole. M23: (E,E)-2,4-nonadienal. M44: benzylcarboxaldehyde. M51: (E,E)-3,5-octadien-2-one. M46: 2-methylnaphthalene. M30: 1-methylnaphthalene. M20: naphthalene. M5: benzaldehyde.
The ‘green’ attribute was positively correlated to 13 compounds including decanal (M45), nonanal (M16), heptaldehyde (M4), (E,E)-2,4-nonadienal (M23), benzylcarboxaldehyde (M44), benzaldehyde (M5), naphthalene (M20), 1-methylnaphthalene (M30), 2-methylnaphthalene (M46), (E,E)-3,5-octadien-2-one (M51), 2-pentylfuran (M43), 4-methylanisole (M53) and dihydroactinolide (M40). Previous study has shown that most of aldehydes were generally associated with ‘green’ attributes (Xu et al., 2007). The ‘floral and fruity’ attribute was significantly positive related to 3,4-dehydro-β-ionone (M38), dihydro-β-ionone (M36), (+)-carotol (M42) and linalool oxide Ⅱ (M14). It is well known that ketone compounds are very important volatile compounds in various teas, providing a special floral attribute, and appear to be the unique volatile compounds in Fu brick tea (Lv et al., 2014). The ‘woody’ and ‘stale’ attributes were only significantly related to α-terpineol (M21). α-Terpineol is a tertiary monoterpenoid alcohol, which could be produced by many microorganisms and was also considered to be an important component of the basic aroma of dark tea (Nie et al., 2019, Sales et al., 2020). In addition, it should be noted that ‘herbal’ attribute is located in the inner ellipse, which indicated that this attribute was not well described by the PLS model, perhaps due to the masking interactions with other attributes.
4. Conclusion
In summary, Fu brick tea could be divided into three aroma types by QDA analysis according to the differences of aroma characteristics, including floral-fungal aroma type, ripe-fungal aroma type and fresh-fungal aroma type. Among them, floral-fungal aroma type of Fu brick tea had strong ‘floral and fruity’ attribute, ripe-fungal aroma type with strong ‘stale’ and ‘woody’ aroma attributes, and green-fungal aroma type with strong significant ‘green’ attribute. A total of 112 volatile compounds were identified and quantified in Fu brick tea samples by HS-SPME/GC–MS analysis. According to OAV analysis, 54 voaltile compounds were identified as aroma compounds. Combined with OPLS-DA analysis, ten aroma compounds dominated with floral and fruity attributes in JHX type samples, eleven aroma compounds dominated with woody, stale and fatty attributes in SJX type samples, and eighteen aroma compounds dominated with green attribute in QJX type samples were identified as key aroma compounds that were responsible for the different aroma characteristics formation in the three aroma types of Fu brick tea. Furthermore, 3,4-dehydro-β-ionone, dihydro-β-ionone, (+)-carotol and linalool oxide Ⅱ were identified as key contributors to ‘floral and fruity’ attribute, ‘α-terpineol was contributed to ‘woody’ and ‘stale’ attributes, and thirteen aroma compounds that mainly composed of aldehydes were related to ‘green’ attribute by PLS analysis. This study provided the useful information for understanding the formation mechanisms of different aroma characteristic in Fu brick tea. Further research can focus on whether the volatile compounds with low OAVs have contribution to the aroma characteristics formation and the interaction of these aroma compounds in the different aroma types of Fu brick tea.
CRediT authorship contribution statement
Zheng Xuexue: Investigation, Visualization, Software, Writing – original draft. Hong Xin: Investigation, Visualization, Software, Writing – original draft. Jin Youlan: Investigation, Methodology, Software, Validation, Resources. Wang Chao: Validation, Writing – review & editing. Liu Zhonghua: Resources, Writing – review & editing. Huang Jianan: Conceptualization, Investigation, Writing – review & editing, Project administration, Funding acquisition. Li Qin: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31871764); and the China Tea Research System Project (CARS-23); and the Open Project Fund of College of Horticulture of Hunan Agricultural University (2021YYXK003); and the “1515 Talent Project” of Hunan Agricultural University.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2022.100248.
Contributor Information
Huang Jianan, Email: jianan-huang@hotmail.com.
Li Qin, Email: liqinvip@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Cao L., Guo X., Liu G., Song Y., Ho C.-T., Hou R.…Wan X. A comparative analysis for the volatile compounds of various Chinese dark teas using combinatory metabolomics and fungal solid-state fermentation. Journal of Food and Drug Analysis. 2018;26(1):112–123. doi: 10.1016/j.jfda.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Chen D., Jiang H., Sun H., Zhang C., Zhao H.…Xu Z. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography-mass spectrometry and olfactometry and sensory analysis. Food Chemistry. 2019;274:130–136. doi: 10.1016/j.foodchem.2018.08.124. [DOI] [PubMed] [Google Scholar]
- Du L.P., Wang C., Li J.X., Xiao D.G., Li C.W., Xu Y.Q. Optimization of Headspace Solid-Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry for Detecting Methoxyphenolic Compounds in Pu-erh Tea. Journal of Agricultural and Food Chemistry. 2013;61(3):561–568. doi: 10.1021/jf304470k. [DOI] [PubMed] [Google Scholar]
- Demyttenaere J.C., Willemen H.M. Biotransformation of linalool to furanoid and pyranoid linalool oxides by Aspergillus niger. Phytochemistry. 1998;47(6):1029–1036. doi: 10.1016/s0031-9422(97)00688-2. [DOI] [PubMed] [Google Scholar]
- Díaz-Maroto M.C., Castillo N., Castro-Vázquez L., Ángel González-Viñas M., Pérez-Coello M.S. Volatile composition and olfactory profile of pennyroyal (Mentha pulegium L.) plants. Flavour and Fragrance Journal. 2007;22(2):114–118. doi: 10.1002/(ISSN)1099-102610.1002/ffj.v22:210.1002/ffj.1766. [DOI] [Google Scholar]
- Dudai N., Weinstein Y., Krup M., Rabinski T., Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Medica. 2005;71(5):484–488. doi: 10.1055/s-2005-864146. [DOI] [PubMed] [Google Scholar]
- Fu D.H., Ryan E.P., Huang J.A., Liu Z.H., Weir T.L., Snook R.L., Ryan T.P. Fermented Camellia sinensis, Fu Zhuan Tea, regulates hyperlipidemia and transcription factors involved in lipid catabolism. Food Research International. 2011;44(9):2999–3005. doi: 10.1016/j.foodres.2011.07.008. [DOI] [Google Scholar]
- Greger V., Schieberle P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. Journal of Agricultural and Food Chemistry. 2007;55(13):5221–5228. doi: 10.1021/jf0705015. [DOI] [PubMed] [Google Scholar]
- Guo X., Ho C.-T., Wan X., Zhu H., Liu Q., Wen Z. Changes of volatile compounds and odor profiles in Wuyi rock tea during processing. Food Chemistry. 2021;341:128230. doi: 10.1016/j.foodchem.2020.128230. [DOI] [PubMed] [Google Scholar]
- Huang Y.H., Wang J., Zeng Z., Lai X.F. Analysis of aroma constituents of fuzhuan tea produced in different years. Food Science. 2011;32(24):261–266. [Google Scholar]
- Kang S., Yan H., Zhu Y., Liu X.u., Lv H.-P., Zhang Y.…Lin Z. Identification and quantification of key odorants in the world's four most famous black teas. Food Research International. 2019;121:73–83. doi: 10.1016/j.foodres.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Li H.H., Luo L.Y., Wang J., Fu D.H., Zeng L. Lexicon development and quantitative descriptive analysis of Hunan fuzhuan brick tea infusion. Food Research International. 2019;120:275–284. doi: 10.1016/j.foodres.2019.02.047. [DOI] [PubMed] [Google Scholar]
- Li M.Y., Xiao Y., Zhong K., Bai J.R., Wu Y.P., Zhang J.Q., Gao H. Characteristics and chemical compositions of Pingwu Fuzhuan brick-tea, a distinctive post-fermentation tea in Sichuan province of China. International Journal of Food Properties. 2019;22(1):878–889. doi: 10.1080/10942912.2019.1614951. [DOI] [Google Scholar]
- Li Q., Li Y., Luo Y.u., Xiao L., Wang K., Huang J., Liu Z. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. Lwt-Food Science and Technology. 2020;127:109355. doi: 10.1016/j.lwt.2020.109355. [DOI] [Google Scholar]
- Li Q., Liu Z.H., Huang J.A., Luo G.A., Liang Q.L., Wang D.…Hu J.H. Anti-obesity and hypolipidemic effects ofFuzhuan brick tea water extract in high-fat diet-induced obese rats. Journal of the Science of Food and Agriculture. 2013;93(6):1310–1316. doi: 10.1002/jsfa.5887. [DOI] [PubMed] [Google Scholar]
- Ling T.J., Wan X.C., Ling W.W., Zhang Z.Z., Xia T., Li D.X., Hou R.Y. New triterpenoids and other constituents from a special microbial-fermented tea-fuzhuan brick tea. Journal of Agricultural and Food Chemistry. 2010;58(8):4945. doi: 10.1021/jf9043524. [DOI] [PubMed] [Google Scholar]
- Liu D.H., Zhou G.H., Xu X.L. “ROAV” method: A new method for determining key odor compounds of Rugao Ham. Food Science. 2008;07:370–374. [Google Scholar]
- Liu X., Liu Y., Li P., Yang J., Wang F., Kim E.…Tu Y. Chemical characterization of Wuyi rock tea with different roasting degrees and their discrimination based on volatile profiles. Rsc Advances. 2021;11(20):12074–12085. doi: 10.1039/D0RA09703A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H.P., Zhong Q.S., Lin Z., Wang L., Tan J.F., Guo L. Aroma characterisation of Pu-erh tea using headspace-solid phase microextraction combined with GC/MS and GC-olfactometry. Food Chemistry. 2012;130(4):1074–1081. doi: 10.1016/j.foodchem.2011.07.135. [DOI] [Google Scholar]
- Lv S.D., Wu Y.S., Li C.W., Xu Y.Q., Liu L., Meng Q.X. Comparative analysis of pu-erh and fuzhuan teas by fully automatic headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry and chemometric methods. Journal of Agricultural and Food Chemistry. 2014;62(8):1810–1818. doi: 10.1021/jf405237u. [DOI] [PubMed] [Google Scholar]
- Ma S.C., Wang M.Q., Liu C.M., Ma W.J., Zhu Y., Lin Z., Lv H.P. Analysis of volatile composition and key aroma compounds of liupao tea. Food Science. 2020;41(20):191–197. doi: 10.7506/spkx1002-6630-20190920-252. [DOI] [Google Scholar]
- Mao S., Lu C., Li M., Ye Y., Wei X.u., Tong H. Identification of key aromatic compounds in Congou black tea by partial least-square regression with variable importance of projection scores and gas chromatography-mass spectrometry/gas chromatography-olfactometry. Journal of the Science of Food and Agriculture. 2018;98(14):5278–5286. doi: 10.1002/jsfa.2018.98.issue-1410.1002/jsfa.9066. [DOI] [PubMed] [Google Scholar]
- Mo H.Z., Zhang H., Li Y.Q., Zhu Y. Antimicrobial activity of the indigenously microbial fermented Fuzhuan brick-tea. Journal of Biotechnology. 2008;136:S722. doi: 10.1016/j.jbiotec.2008.07.1719. [DOI] [Google Scholar]
- Nie C.N., Zhong X.X., He L., Gao Y., Zhang X., Wang C.M., Du X. Comparison of different aroma-active compounds of Sichuan Dark brick tea (Camellia sinensis) and Sichuan Fuzhuan brick tea using gas chromatography-mass spectrometry (GC-MS) and aroma descriptive profile tests. European Food Research and Technology. 2019;245(9):1963–1979. doi: 10.1007/s00217-019-03304-1. [DOI] [Google Scholar]
- Sales A., Felipe L.d.O., Bicas J.L. Production, properties, and applications of alpha-terpineol. Food and Bioprocess Technology. 2020;13(8):1261–1279. doi: 10.1007/s11947-020-02461-6. [DOI] [Google Scholar]
- Shi J., Zhu Y., Zhang Y., Lin Z., Lv H.P. Volatile composition of Fu-brick tea and Pu-erh tea analyzed by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Lwt-Food Science and Technology. 2019;103:27–33. doi: 10.1016/j.lwt.2018.12.075. [DOI] [Google Scholar]
- Shi Y., Wang M., Dong Z., Zhu Y., Shi J., Ma W.…Lv H. Volatile components and key odorants of Chinese yellow tea (Camellia sinensis) Lwt-Food Science and Technology. 2021;146:111512. doi: 10.1016/j.lwt.2021.111512. [DOI] [Google Scholar]
- Tanguler H., Selli S., Sen K., Cabaroglu T., Erten H. Aroma composition of shalgam: A traditional Turkish lactic acid fermented beverage. Journal of Food Science and Technology-Mysore. 2017;54(7):2011–2019. doi: 10.1007/s13197-017-2637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.Q., Ma W.J., Shi J., Zhu Y., Lin Z., Lv H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Research International. 2020;130:108908. doi: 10.1016/j.foodres.2019.108908. [DOI] [PubMed] [Google Scholar]
- Wang H., Hua J., Jiang Y., Yang Y., Wang J., Yuan H. Influence of fixation methods on the chestnut-like aroma of green tea and dynamics of key aroma substances. Food Research International. 2020;136:109479. doi: 10.1016/j.foodres.2020.109479. [DOI] [PubMed] [Google Scholar]
- Wang X.Q., Zeng L.T., Liao Y.Y., Zhou Y., Xu X.L., Dong F., Yang Z.Y. An alternative pathway for the formation of aromatic aroma compounds derived from L-phenylalanine via phenylpyruvic acid in tea (Camellia sinensis (L.) O. Kuntze) leaves. Food Chemistry. 2019;270:17–24. doi: 10.1016/j.foodchem.2018.07.056. [DOI] [PubMed] [Google Scholar]
- Xu A.Q., Wang Y.L., Wen J.Y., Liu P., Liu Z.Y., Li Z.J. Fungal community associated with fermentation and storage of Fuzhuan brick-tea. International Journal of Food Microbiology. 2011;146(1):14–22. doi: 10.1016/j.ijfoodmicro.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Xu X., Mo H., Yan M., Zhu Y. Analysis of characteristic aroma of fungal fermented Fuzhuan brick-tea by gas chromatography/mass spectrophotometry. Journal of the Science of Food and Agriculture. 2007;87(8):1502–1504. doi: 10.1002/(ISSN)1097-001010.1002/jsfa.v87:810.1002/jsfa.2874. [DOI] [Google Scholar]
- Xu Y.Q., Wang C., Li C.Q., Liu S.H., Zhang C.X., Li L.W., Jiang D.H. characterization of aroma-active compounds of Pu-erh Tea by headspace solid-phase microextraction (HS-SPME) and simultaneous distillation-extraction (SDE) coupled with GC-olfactometry and GC-MS. Food Analytical Methods. 2016;9(5):1188–1198. doi: 10.1007/s12161-015-0303-7. [DOI] [Google Scholar]
- Zhang H., Wang J., Zhang D., Zeng L., Liu Y., Zhu W.…Huang Y. Aged fragrance formed during the post-fermentation process of dark tea at an industrial scale. Food Chemistry. 2021;342:128175. doi: 10.1016/j.foodchem.2020.128175. [DOI] [PubMed] [Google Scholar]
- Zhang W., Si G., Du H., Li J., Zhou P., Ye M. Directional design of a starter to assemble the initial microbial fermentation community of baijiu. Food Research International. 2020;134:109255. doi: 10.1016/j.foodres.2020.109255. [DOI] [PubMed] [Google Scholar]
- Zhao R.L., Xu W., Wu D., Jiang Y.H., Zhu Q. Comparison of the sensory characteristics and aroma components of Fu brick tea for sale in border areas of different regions. Modern Food Science and Technology. 2017;33(10):217–224. doi: 10.13982/j.mfst.1673-9078.2017.10.031. [DOI] [Google Scholar]
- Zhu H., Qiu J., Li Z.G. Determination of rheological property and its effect on key aroma release of Shanxi aged vinegar. Journal of Food Science and Technology-Mysore. 2016;53(8):3304–3311. doi: 10.1007/s13197-016-2305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J.C., Chen F., Wang L.Y., Niu Y.W., Yu D., Shu C.…Xiao Z.B. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. Journal of Agricultural and Food Chemistry. 2015;63(34):7499–7510. doi: 10.1021/acs.jafc.5b02358. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Lv H.P., Shao C.Y., Kang S., Zhang Y., Guo L.…Lin Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Research International. 2018;108:74–82. doi: 10.1016/j.foodres.2018.03.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.