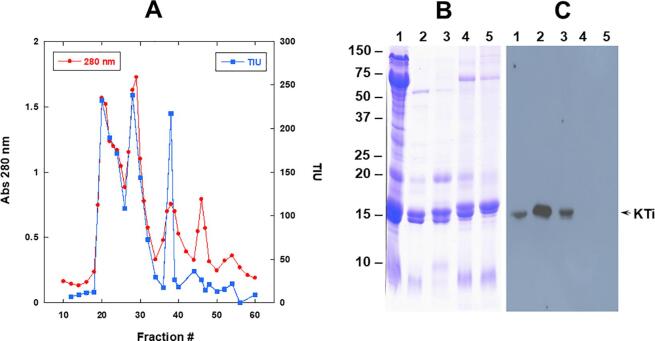
Fig. 2.
Separation of A. pavonina seed proteins by column chromatography and detection of trypsin inhibitors by immunoblot analysis. Panel A. A partially purified protein fraction was loaded on an ion-exchange DE-52 cellulose column. Unbound proteins were removed by washing the column with excess 100 mM Tris-HCl, pH 8.0 buffer. DE-52 cellulose bound proteins were eluted with a step gradient of sodium chloride (0–500 mM M) and 5 mL fractions were collected. Absorbance at 280 nm (0--0) and trypsin inhibitor activity (□----□) in alternate fractions were measured. Total seed protein and DE-52 peak A, B, C and D fractions were separated on a 15% SDS-PAGE gels. Resolved proteins were stained with Coomassie Blue (Panel B) or transferred to nitrocellulose membrane and incubated with soybean Kunitz trypsin inhibitor antibodies (Panel C). Immunoreactive proteins were detected by chemiluminescence using anti-rabbit IgG horseradish peroxidase conjugate. Lane 1, total protein; lane 2, DE-52 peak A; lane 3, DE-52 peak B; lane 4, DE-52 peak C; and lane 5, DE-52 peak D. The position and sizes of protein markers in kDa are shown on the left side of the figure. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)