Abstract
Background
Obesity and cardiometabolic dysfunction have been associated with cancer risk and severity. Underlying mechanisms remain unclear.
Objectives
The aim of this study was to examine associations of obesity and related cardiometabolic traits with incident cancer.
Methods
FHS (Framingham Heart Study) and PREVEND (Prevention of Renal and Vascular End-Stage Disease) study participants without prevalent cancer were studied, examining associations of obesity, body mass index (BMI), waist circumference, visceral adipose tissue (VAT) and subcutaneous adipose tissue depots, and C-reactive protein (CRP) with future cancer in Cox models.
Results
Among 20,667 participants (mean age 50 years, 53% women), 2,619 cancer events were observed over a median follow-up duration of 15 years. Obesity was associated with increased risk for future gastrointestinal (HR: 1.30; 95% CI: 1.05-1.60), gynecologic (HR: 1.62; 95% CI: 1.08-2.45), and breast (HR: 1.32; 95% CI: 1.05-1.66) cancer and lower risk for lung cancer (HR: 0.62; 95% CI: 0.44-0.87). Similarly, waist circumference was associated with increased risk for overall, gastrointestinal, and gynecologic but not lung cancer. VAT but not subcutaneous adipose tissue was associated with risk for overall cancer (HR: 1.22; 95% CI: 1.05-1.43), lung cancer (HR: 1.92; 95% CI: 1.01-3.66), and melanoma (HR: 1.56; 95% CI: 1.02-2.38) independent of BMI. Last, higher CRP levels were associated with higher risk for overall, colorectal, and lung cancer (P < 0.05 for all).
Conclusions
Obesity and abdominal adiposity are associated with future risk for specific cancers (eg, gastrointestinal, gynecologic). Although obesity was associated with lower risk for lung cancer, greater VAT and CRP were associated with higher lung cancer risk after adjusting for BMI.
Key Words: epidemiology, gastrointestinal cancer, inflammation, obesity, risk factor
Abbreviations and Acronyms: BMI, body mass index; CRP, C-reactive protein; CT, computed tomographic; CVD, cardiovascular disease; HOMA-IR, homeostatic model assessment of insulin resistance; PAI, plasminogen activator inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; WC, waist circumference
Central Illustration
Cardiometabolic risk factors, in particular obesity, are known risk factors for many highly burdensome diseases, including diabetes, cardiovascular disease (CVD), cancer, and death.1,2 Obesity is increasing globally, with more than 40% of adults and 20% of children in the United States currently categorized as obese (body mass index [BMI] ≥30 kg/m2) and 6.9% of incident cancers attributed to excess body weight.3, 4, 5 Increased adiposity creates a chronic proinflammatory state, which may be a shared mechanism underlying the development of many comorbidities, including cancer.6, 7, 8 Additionally, obesity may alter the tumor microenvironment and promote tumor progression via complex immunologic mechanisms.9 Other putative mechanisms for obesity and cancer risk include adipose production of excess estrogen, hyperinsulinemia or insulin resistance, increased production of adipokines, and direct or indirect effects on cell growth regulators.6,10 Epidemiologic studies have long shown an association between obesity and incident cancer, and there is consensus among the National Cancer Institute and World Health Organization that 13 cancer types are associated with excess body weight, including breast, female reproductive, and colon cancers.11, 12, 13, 14
Retrospective electronic health record–based and registry-based studies, however, may introduce selection and ascertainment bias. Furthermore, there is broad agreement that although BMI is a widely available measurement used to categorize obesity, it may not accurately reflect adiposity across age, sex, race, and muscle composition.15 For example, abdominal adiposity measured by waist circumference (WC) has been more closely associated with CVD risk factors16 and may impart additional valuable information compared with BMI alone in understanding the association between obesity and cancer.17 In this context, we sought to leverage 2 large prospective longitudinal community-based inception cohorts to examine the association of obesity with future development of histologically confirmed cancer. Moreover, we sought to examine detailed phenotyping of obesity-related traits, including anthropometrics, advanced imaging to quantify adipose tissue depots including visceral adiposity as a known source of inflammatory mediators, and circulating biomarker evidence of putative mechanisms linking obesity to cancer risk.
Methods
Study sample
We studied participants in the FHS (Framingham Heart Study) and the PREVEND (Prevention of Renal and Vascular End-Stage Disease) study, 2 prospective community-based cohorts.18, 19, 20, 21 We included FHS participants who attended Original Cohort examination 16 (1979-1982) or 24 (1995-1998), Offspring Cohort examination 2 (1979-1983) or 6 (1995-1998), and Third Generation Cohort examination 1 (2002-2005) and PREVEND participants who attended the first examination (1997-1998). We excluded individuals with prevalent cancer (except for nonmelanoma skin cancer; n = 934), prevalent heart failure (n = 185), prevalent myocardial infarction (n = 800), end-stage renal disease (n = 52), missing covariates (n = 625), and missing follow-up data (n = 1), leaving a final sample of 20,667 participants (see Supplemental Figure 1 for details). The study was approved by the appropriate Institutional Review Boards, and all participants provided written informed consent.
Study procedures
At baseline examination, study participants underwent medical history review, physical examination, anthropometry, and collection of fasting blood as described previously.18, 19, 20, 21 BMI was calculated as weight (kilograms) divided by height (meters) squared. Obesity is defined as BMI ≥30 kg/m2. Current smoking was defined as smoking 1 or more cigarettes per day over the past year. Fasting circulating inflammatory biomarkers, glucose, and insulin were ascertained among FHS participants at Offspring Cohort examination 6 and PREVEND (N = 10,995) (details are provided in the Supplemental Appendix).
A subset of FHS participants attending Offspring Cohort examination 7 (1998-2001) and Third Generation Cohort examination 1 (N = 3,077) underwent multidetector computed tomographic (CT) assessment of various adipose depots as previously described (LightSpeed Ultra, GE Medical Systems; details are provided in the Supplemental Appendix).22,23 Intrareader and interreader reproducibility were excellent (intrareader intraclass correlation coefficients: visceral adipose tissue [VAT], 0.99; subcutaneous adipose tissue [SAT], 0.99; intrathoracic fat, 0.99; and pericardial fat, 0.97; interreader intraclass correlation coefficients: VAT, 0.992; SAT, 0.997; intrathoracic fat, 0.98; and pericardial fat, 0.95).23,24
Ascertainment of cancer outcomes
Participants were followed prospectively for the occurrence of incident cancer. In FHS, cancer cases were identified through surveillance of routine examinations, health updates, hospital admissions, or death records through December 31, 2016. We reviewed medical records and pathology reports, and cancer cases were adjudicated and coded on the basis of topology and morphology and graded by 2 independent physicians using International Classification of Diseases codes.25 In PREVEND, cancer cases were identified through linkage with the Dutch nationwide network and registry of histopathology and cytopathology in the Netherlands, Pathologisch-Anatomisch Landelijk Geautomatiseerd Archief Foundation (https://www.palga.nl/).26 All malignancies except nonmelanoma skin cancers were included for analysis. Site-specific cancers included gastrointestinal, colorectal (a subset of gastrointestinal cancers), lung cancer, melanoma, hematologic, bladder, prostate, breast, and gynecologic cancers (additional details are provided in the Supplemental Appendix).
Statistical analysis
Baseline characteristics are reported as mean ± SD, median (IQR), or percentages as appropriate. The primary outcome was incident cancer, and secondary analyses examined time to incident site-specific cancers. Gynecologic and breast cancer analyses were restricted to women, and prostate cancer analyses were restricted to men. We plotted cumulative cancer incidence by BMI and sex-specific WC quartiles using the Fine and Gray method. To highlight parallel associations with incident CVD (defined as myocardial infarction, stroke, or heart failure), we similarly plotted cumulative CVD incidence across BMI and WC quartiles. Using multivariable cause-specific Cox models to examine potential etiology of disease, we examined associations of BMI and WC with incident cancer, and effect estimates were presented per 1-SD difference. Cox models were adjusted for age, sex, diabetes mellitus, systolic blood pressure, hypertension treatment, smoking status (current, former, or never), and ratio of total cholesterol to high-density lipoprotein. These covariates were chosen a priori on the basis of prior associations with cancer or cancer subtypes.2 There was no evidence of multicollinearity in our models, and restricted cubic splines revealed no evidence of a nonlinear association of BMI and cancer. Additional sensitivity analyses was performed using the Fine and Gray method to derive the subdistribution hazards.
In secondary analyses, we examined the association of adipose depots, C-reactive protein (CRP), plasminogen activator inhibitor (PAI)–1, and homeostatic model assessment of insulin resistance (HOMA-IR) with cancer incidence in multivariable Cox models with and without the addition of BMI. Biomarkers were natural log transformed because of right-skewed distributions and effect sizes expressed per 1-SD change in log unit. Exploratory analyses including sex interactions, stratified analyses by smoking status, and lag time analyses are detailed in the Supplemental Appendix.
Follow-up was censored at the time of first cancer event, death, or 15 years of follow-up. For site-specific cancer analyses, participants who developed 1 subtype of cancer distinct from the outcome of interest were still considered at risk for subsequent development of the cancer subtype of interest. Analyses accounted for cohort as a strata variable. A 2-sided P value <0.05 was considered to indicate statistical significance. Cox models met proportional hazards assumption. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
We studied 20,667 participants from the FHS and PREVEND cohorts (mean age 50.3 ± 14.2 years, 53% women) who were free from cancer at baseline examination. Prevalent comorbidities included 4% with diabetes, 32% with hypertension, 19% with obesity (BMI ≥30 kg/m2), and 28% with current and 33% with former smoking. Clinical characteristics are displayed by obesity status in Table 1, with greater comorbid burden among obese compared with nonobese individuals. For stratification by cohort, CT imaging, and biomarker availability, see Supplemental Tables 1 to 3.
Table 1.
Baseline Characteristics by Obesity Status
| Overall (n = 20,667) | Obesea (n = 3,924 [19%]) | Nonobese (n = 16,743 [81%]) | |
|---|---|---|---|
| Age, y | 50 ± 14 | 53 ± 13 | 50 ± 14 |
| Women | 11,024 (53) | 2,023 (52) | 9,001 (54) |
| Body mass index, kg/m2 | 26.5 ± 4.8 | 33.9 ± 4.0 | 24.7 ± 2.9 |
| Systolic BP, mm Hg | 126 ± 19 | 133 ± 19 | 125 ± 19 |
| Diastolic BP, mm Hg | 75 ± 10 | 79 ± 10 | 75 ± 10 |
| Total cholesterol, mg/dL | 209 ± 42 | 214 ± 41 | 208 ± 43 |
| HDL cholesterol, mg/dL | 52 ± 16 | 45 ± 13 | 53 ± 16 |
| Triglycerides, mg/dL | 104 (74-153) | 137 (98-197) | 97 (70-142) |
| Diabetes | 894 (4) | 407 (10) | 487 (3) |
| Hypertension | 6,572 (32) | 1,969 (50) | 4,603 (27) |
| Current smoking | 5,876 (28) | 951 (24) | 4,925 (29) |
| Former smoking | 6,918 (33) | 1,435 (37) | 5,483 (33) |
Values are mean ± SD, n (%), or median (IQR).
BP = blood pressure; HDL = high-density lipoprotein.
Obesity is defined as body mass index ≥ 30 kg/m2.
Association of BMI and WC with incident cancer
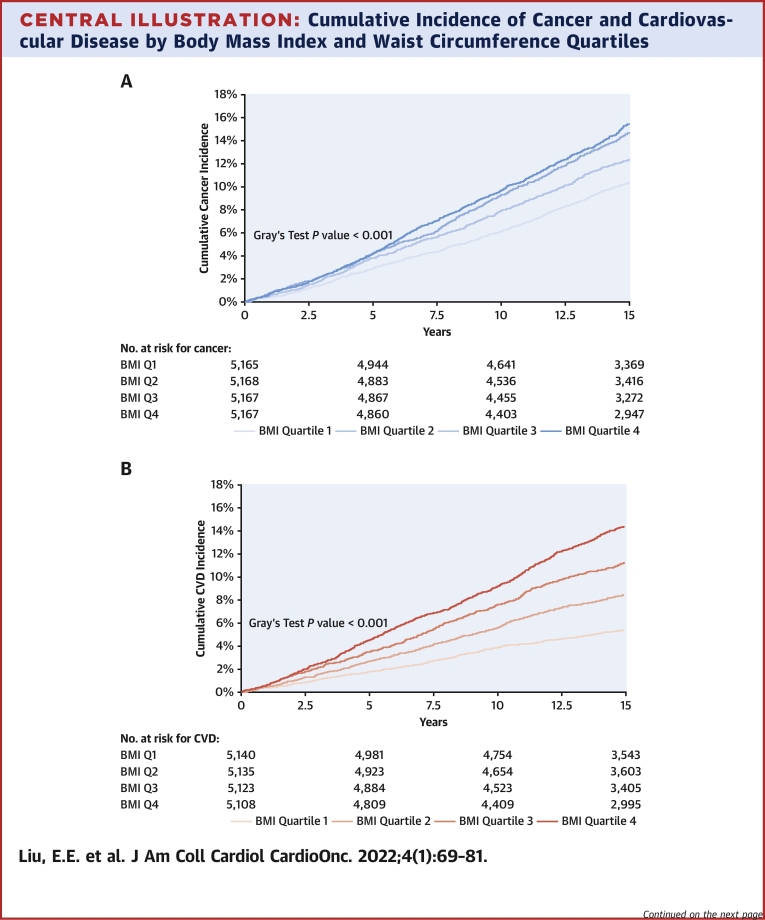
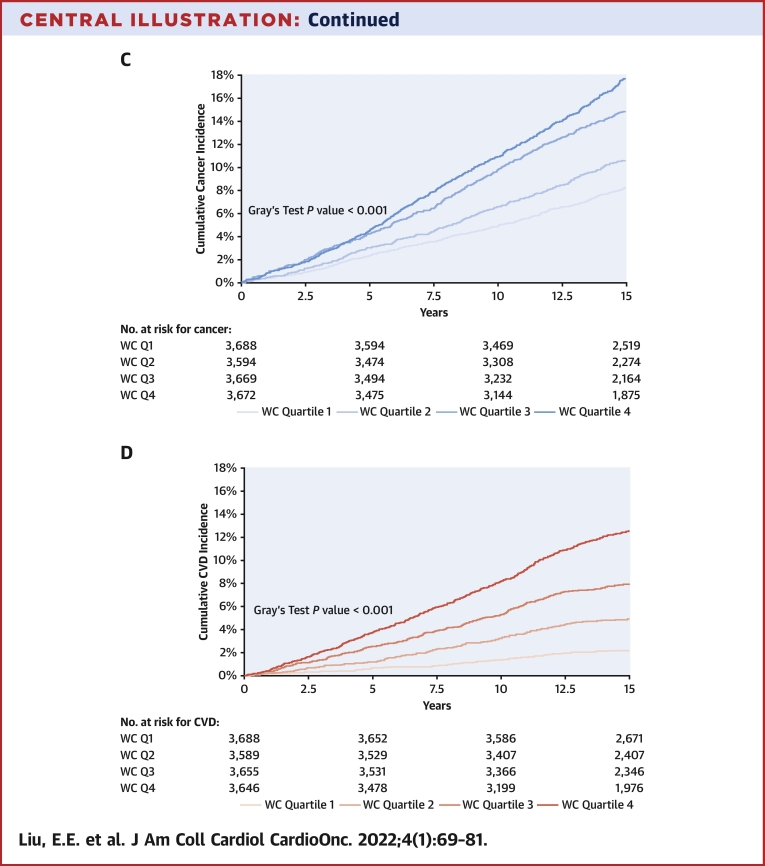
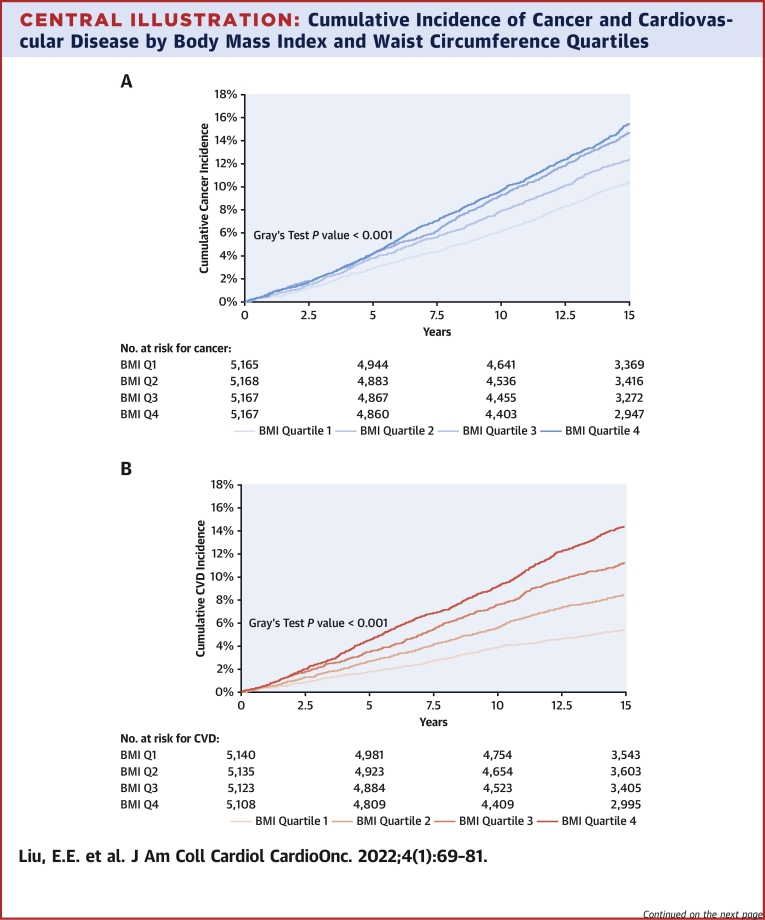
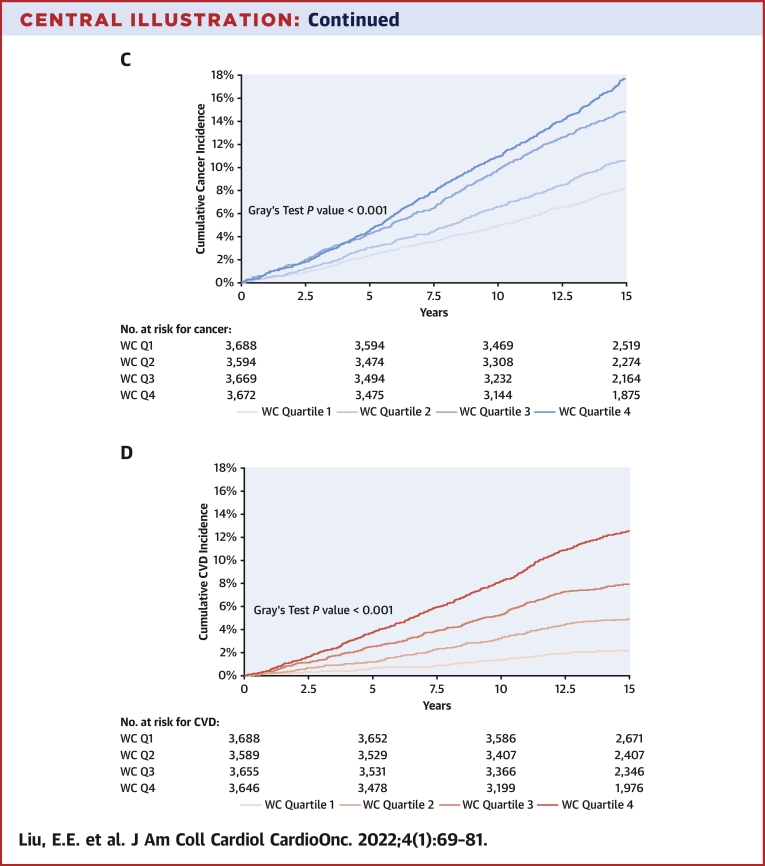
We observed 2,619 incident cancers over a median follow-up period of 15 years (IQR: 13-15 years), including 165 melanomas, 534 gastrointestinal (338 of which were colorectal), 295 lung, 194 hematologic, 176 bladder, 408prostate, 455 breast, and 128 gynecologic cancers. Over that same time period, there were 1,966 CVD events. In cumulative incidence plots, we observed increasing risk for future cancer across higher BMI quartiles and similar trends across higher WC quartiles over 15 years of follow-up. In parallel, we observed increasing cumulative incidence of CVD across higher BMI and WC quartiles (Central Illustration) (P < 0.001 for both, Gray’s test). The incidence rate of overall cancer was 9.5 per 1,000 person-years, and that of CVD was 7.1 per 1,000 person-years.
Central Illustration.
Cumulative Incidence of Cancer and Cardiovascular Disease by Body Mass Index and Waist Circumference Quartiles
The cumulative incidence of all-cause cancer over 15 years of follow-up is displayed by body mass index (BMI) quartiles (A) and by sex-specific waist circumference (WC) quartiles (C). The cumulative incidence of cardiovascular disease over 15 years of follow-up is displayed by BMI quartiles (B) and by WC quartiles (D). BMI quartile cutpoints were as follows: 25th percentile, 23.2 kg/m2; 50th percentile, 25.8 kg/m2; and 75th percentile, 28.9 kg/m2. WC quartile cutpoints for women were as follows: 25th percentile, 76 cm; 50th percentile, 84.2 cm; and 75th percentile, 94.9 cm. WC quartile cutpoints for men were as follows: 25th percentile, 88.3 cm; 50th percentile, 95.3 cm; and 75th percentile, 103.5 cm. BMI = Body Mass Index; CVD = Cardiovascular Disease; Q1 = Quartile 1; Q2 = Quartile 2; Q3 = Quartile 3; Q4 =Quartile 4; WC = Waist Circumference.
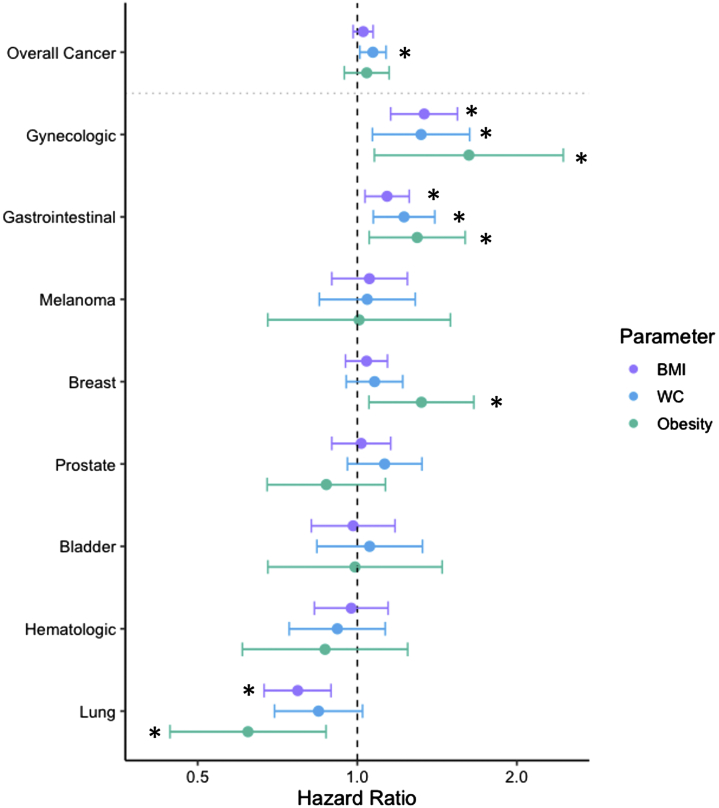
In multivariable Cox models, BMI as a continuous variable and obesity status were both associated with gastrointestinal, gynecologic, and lung cancers but not overall cancer (Tables 2 and 3, Figure 1). Specifically, a 1-SD higher BMI was associated with 14% increased risk for gastrointestinal and colorectal cancers (HR: 1.14 [95% CI: 1.03-1.25; P = 0.008] for gastrointestinal cancer; HR: 1.14 [95% CI: 1.01-1.28; P = 0.040] for colorectal cancer) and 34% increased risk for gynecologic cancer (HR: 1.34; 95% CI: 1.16-1.55; P < 0.001). In contrast, a 1-SD higher BMI was associated with 23% lower risk for lung cancer (HR: 0.77; 95% CI: 0.67-0.89; P < 0.001), an association that appeared linear across the BMI range when examining a restricted cubic spline. Obesity status, defined as BMI ≥30 kg/m2, was associated with increased risk for incident gastrointestinal (HR: 1.30; 95% CI: 1.05-1.60), gynecologic (HR: 1.62; 95% CI: 1.08-2.45), and breast (HR: 1.32; 95% CI: 1.05-1.66) cancer, and lower risk for lung cancer (HR: 0.62; 95% CI: 0.44-0.87) (Table 3).
Table 2.
BMI and WC Multivariable Associations With Cancer Incidence
| BMI (No. at Risk = 20,667) |
WC (No. at Risk = 14,623) |
|||||
|---|---|---|---|---|---|---|
| Events | HR (95% CI) | P Value | Events | HR (95% CI) | P Value | |
| Overall cancer | 2,619 | 1.03 (0.98-1.07) | 0.25 | 1,789 | 1.07 (1.01-1.13) | 0.020 |
| GI cancer | 534 | 1.14 (1.03-1.25) | 0.008 | 328 | 1.22 (1.07-1.40) | 0.003 |
| Colorectal cancer | 338 | 1.14 (1.01-1.28) | 0.040 | 206 | 1.21 (1.02-1.43) | 0.027 |
| Lung cancer | 295 | 0.77 (0.67-0.89) | <0.001 | 182 | 0.85 (0.70-1.02) | 0.084 |
| Melanoma | 165 | 1.05 (0.89-1.24) | 0.53 | 132 | 1.04 (0.85-1.29) | 0.68 |
| Hematologic cancer | 194 | 0.97 (0.83-1.14) | 0.75 | 142 | 0.92 (0.74-1.13) | 0.41 |
| Bladder cancer | 176 | 0.98 (0.82-1.18) | 0.84 | 129 | 1.07 (0.84-1.33) | 0.65 |
| Prostate cancer | 408 | 1.02 (0.89-1.16) | 0.80 | 283 | 1.13 (0.96-1.32) | 0.15 |
| Breast cancer | 455 | 1.04 (0.95-1.14) | 0.39 | 320 | 1.08 (0.95-1.22) | 0.23 |
| Gynecologic cancer | 128 | 1.34 (1.16-1.55) | <0.001 | 93 | 1.32 (1.07-1.63) | 0.010 |
BMI and WC are standardized. Multivariable model is adjusted for age, sex, diabetes mellitus, systolic blood pressure, hypertension treatment, smoking status, and cholesterol ratio. Effect sizes for BMI and WC were expressed per 1-SD change. P values <0.05 were considered to indicate statistical significance. SDs for BMI and WC are 4.8 kg/m2 and 14.3 cm, respectively.
BMI = body mass index; GI = gastrointestinal; WC = waist circumference.
Table 3.
Obesity Multivariable Associations With Cancer Incidence
| Events | HR (95% CI) | P Value | |
|---|---|---|---|
| Overall cancer | 2,619 | 1.04 (0.95-1.15) | 0.41 |
| Gastrointestinal cancer | 534 | 1.30 (1.05-1.60) | 0.014 |
| Colorectal cancer | 338 | 1.31 (1.01-1.71) | 0.043 |
| Lung cancer | 295 | 0.62 (0.44-0.87) | 0.006 |
| Melanoma | 165 | 1.01 (0.68-1.50) | 0.97 |
| Hematologic cancer | 194 | 0.87 (0.61-1.24) | 0.44 |
| Bladder cancer | 176 | 0.99 (0.68-1.45) | 0.96 |
| Prostate cancer | 408 | 0.87 (0.68-1.13) | 0.30 |
| Breast cancer | 455 | 1.32 (1.05-1.66) | 0.017 |
| Gynecologic cancer | 128 | 1.62 (1.08-2.45) | 0.021 |
Obesity status is dichotomous, and HRs are presented for obesity (defined as body mass index ≥30 kg/m2). Multivariable model is adjusted for age, sex, diabetes mellitus, systolic blood pressure, hypertension treatment, smoking status, and cholesterol ratio. Values of P <0.05 were considered to indicate statistical significance.
Figure 1.
Multivariable-Adjusted Associations of Obesity, BMI, and WC With Incident Cancer
The multivariable Cox model was adjusted for age, sex, diabetes, systolic blood pressure, hypertension treatment, smoking status (current, former, or never), and ratio of total cholesterol to high-density lipoprotein. Gynecologic and breast cancer analyses were performed only in women, and prostate cancer analyses were performed only in men. Effect sizes for body mass index (BMI) and waist circumference (WC) were expressed per 1-SD change. ∗P < 0.05.
We found that higher WC was associated with increased risk for future overall cancer (HR: 1.07; 95% CI: 1.01-1.13; P = 0.020) (Table 2, Figure 1). With respect to site-specific cancer, a 1-SD higher WC was associated with an increased risk for future gastrointestinal cancer, including colorectal cancer (HR: 1.22; 95% CI: 1.07-1.40; P = 0.003), and 32% increased risk for gynecologic cancer (HR: 1.32; 95% CI: 1.07-1.63; P = 0.010). In contrast, WC was not associated with incident lung cancer. Sensitivity analyses using the Fine and Gray method to determine the subdistribution HRs yielded similar associations between cancer type and BMI and WC.
Association of CT measures of adiposity with incident cancer
In secondary analyses, we examined the association of various adipose depots with future incident cancer (Table 4). In multivariable analyses adjusting for BMI in addition to covariates in main analyses, we observed that VAT was associated with overall cancer (HR: 1.22; 95% CI: 1.05-1.43; P = 0.011) as well as certain site-specific cancers. Specifically, 1-SD higher VAT was associated with an almost 2-fold increased risk for lung cancer (HR: 1.92; 95% CI: 1.01-3.65; P = 0.045) and 56% increased risk for melanoma (HR: 1.56; 95% CI: 1.02-2.37; P = 0.038). We found no significant associations with other site-specific cancers.
Table 4.
Computed Tomographic Adipose Depot Multivariable Model Associations With Cancer Incidence
| Visceral Adipose Tissue |
Pericardial Adipose Tissue |
Intrathoracic Adipose Tissue |
Subcutaneous Adipose Tissue |
Liver Phantom Ratio |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Overall cancer (n/N = 394/3,077) | 1.22 (1.05-1.43) | 0.011 | 1.14 (1.02-1.27) | 0.018 | 1.11 (0.97-1.27) | 0.12 | 0.95 (0.79-1.14) | 0.56 | 1.02 (0.92-1.14) | 0.70 |
| Gastrointestinal cancer (n/N = 49/3,077 | 1.11 (0.73-1.68) | 0.62 | 1.08 (0.79-1.47) | 0.62 | 1.33 (0.97-1.83) | 0.078 | 1.47 (0.88-2.43) | 0.14 | 1.07 (0.78-1.46) | 0.67 |
| Colorectal cancer (n/N = 28/3,077) | 1.55 (0.90-2.67) | 0.11 | 1.03 (0.67-1.57) | 0.91 | 1.32 (0.85-2.05) | 0.21 | 1.70 (0.89-3.24) | 0.11 | 0.97 (0.66-1.43) | 0.90 |
| Lung cancer (n/N = 21/3,077) | 1.92 (1.01-3.65) | 0.045 | 1.51 (1.01-2.26) | 0.044 | 1.51 (0.87-2.62) | 0.15 | 1.53 (0.67-3.51) | 0.31 | 0.85 (0.58-1.26) | 0.43 |
| Melanoma (n/N = 54/3,077) | 1.56 (1.02-2.37) | 0.038 | 1.40 (1.07-1.82) | 0.013 | 1.38 (1.00-1.91) | 0.047 | 1.09 (0.65-1.83) | 0.75 | 0.92 (0.68-1.23) | 0.56 |
| Hematologic cancer (n/N = 43/3,077) | 0.96 (0.60-1.54) | 0.88 | 0.76 (0.51-1.12) | 0.17 | 0.70 (0.44-1.11) | 0.13 | 0.91 (0.50-1.64) | 0.74 | 1.31 (0.89-1.93) | 0.18 |
| Bladder cancer (n/N = 30/3,077) | 1.16 (0.71-1.91) | 0.55 | 1.18 (0.84-1.67) | 0.34 | 0.84 (0.52-1.34) | 0.46 | 1.07 (0.50-2.28) | 0.86 | 1.05 (0.71-1.54) | 0.80 |
| Prostate cancer (n/N = 81/1,595) | 1.29 (0.95-1.76) | 0.10 | 1.11 (0.89-1.39) | 0.34 | 1.07 (0.82-1.40) | 0.63 | 1.05 (0.67-1.63) | 0.84 | 0.93 (0.75-1.17) | 0.54 |
| Breast cancer (n/N = 84/1,482) | 1.29 (0.86-1.93) | 0.22 | 1.22 (0.93-1.61) | 0.15 | 1.03 (0.68-1.57) | 0.88 | 0.94 (0.64-1.38) | 0.77 | 0.92 (0.72-1.19) | 0.53 |
| Gynecologic cancer (n/N = 21/1,482) | 1.03 (0.48-2.21) | 0.94 | 0.61 (0.31-1.23) | 0.17 | 1.46 (0.70-3.02) | 0.31 | 0.39 (0.19-0.79) | 0.009 | 1.15 (0.68-1.94) | 0.60 |
Adiposity measures are standardized. Decreasing liver phantom ratio corresponds to a greater amount of liver fat. Multivariable model includes variables from main analyses (age, sex, diabetes mellitus, systolic blood pressure, hypertension treatment, smoking status, and cholesterol ratio) and body mass index. Displayed events (n/N) are those of visceral adipose tissue analyses. Event numerator and denominator vary slightly for other adipose depots analyses. Effect sizes were expressed per 1-SD change. Values of P < 0.05 were considered to indicate statistical significance. SDs for adiposity measures are 1,011.4 cm3 (visceral), 1,390.9 cm3 (subcutaneous), 42.9 cm3 (pericardial), 61.6 cm3 (intrathoracic), and 9.7 (liver phantom ratio).
We found similar associations of pericardial adipose tissue with overall cancer (HR: 1.14; 95% CI: 1.02-1.27; P = 0.018), melanoma (HR: 1.40; 95% CI: 1.07-1.82; P = 0.013), and lung cancer (HR: 1.51; 95% CI: 1.01-2.26; P = 0.044). In contrast, we found no associations of subcutaneous or intrathoracic adipose depots or indexes of liver adiposity with overall cancer or site-specific cancers.
Association of inflammatory biomarkers and HOMA-IR with incident cancer
In multivariable analyses without accounting for BMI, we observed that circulating CRP level was associated with increased risk for overall cancer and gastrointestinal, colorectal, lung, and gynecologic cancers (Supplemental Table 4). Specifically, a 1-SD increase in CRP was associated with a 13% increase in risk for overall cancer (HR: 1.13; 95% CI: 1.07-1.19; P < 0.001). The association of CRP with overall cancer, colorectal cancer, and lung cancer remained significant after further adjusting for BMI, whereas the associations with gynecologic and gastrointestinal cancers were attenuated.
For PAI-1 and HOMA-IR, models with and without BMI showed inconsistent associations with specific cancer outcomes (Supplemental Table 4).
Sensitivity and exploratory analyses
To minimize the potential contribution of undiagnosed cancer cases at baseline, we excluded 129 cancer cases that were diagnosed within 1 year of baseline examination in sensitivity analyses. The results for association of BMI and WC were similar to those of our main analyses (Supplemental Table 5). In exploratory analyses with further adjustment for heavy alcohol use in the multivariable model, results were similar to those of the main analyses (Supplemental Table 6).
In sex-stratified analyses, there was no significant sex × BMI interaction for overall or site-specific cancer. There was, however, a significant sex × WC interaction (Pinteraction = 0.03) for overall cancer (HR: 1.11 [95% CI: 1.02-1.21; P = 0.021] for men; HR: 1.06 [95% CI: 0.98-1.15; P = 0.12] for women) but not site-specific cancers.
To examine the finding of an association of higher BMI with lower lung cancer risk, we stratified analyses by smoking status (current, 166 events per 5,876 participants; former, 107 events per 6,918 participants; never, 22 events per 7,873 participants). We found no significant associations between BMI and lung cancer among never and former smoking groups, though directionality of effect was similar to overall analyses (never smoking: HR: 0.81 [95% CI: 0.49-1.36; P = 0.44]; former smoking: HR: 0.84 [95% CI: 0.66-1.08; P = 0.17]). Higher BMI was associated with lower risk for lung cancer among individuals with current smoking (HR: 0.74; 95% CI: 0.61-0.90; P = 0.003).
Last, to address potential surveillance bias, we examined survival after cancer diagnosis stratified by obese and nonobese individuals. In both Kaplan-Meier survival curves (P for log rank = 0.11) as well as time-to-event analyses (Cox model adjusted for age; HR: 1.02 [95% CI: 0.83-1.25; P = 0.87] comparing obese vs nonobese individuals), we found no differences in postcancer diagnosis survival on the basis of obesity status.
Discussion
We leveraged 2 large longitudinal community-based inception cohorts with histologically confirmed incident cancer cases to examine associations of obesity and related traits with risk for future cancer. First, we found that obesity, defined as BMI ≥30 kg/m2, was associated with future risk for specific cancer subtypes, including approximately 30% greater risk for gastrointestinal and breast cancers and approximately 60% increased risk for gynecologic cancers. Conversely, obesity was associated with approximately 38% lower risk for lung cancer. Second, abdominal adiposity (WC) was associated with gastrointestinal, breast, and overall cancer but not lung cancer. The importance of abdominal adiposity independent of BMI was substantiated in secondary analyses demonstrating that greater VAT but not SAT was associated with overall and lung cancer, as well as melanoma. Last, on interrogation of potential obesity-associated pathways using biomarkers, we found that baseline inflammation (CRP) was associated with greater risk for overall cancer, colorectal, and lung cancer, whereas no such association was observed for insulin resistance (HOMA-IR). Our findings suggest that abdominal adiposity and systemic inflammation are associated with specific cancer subtypes and underscore the need for future studies to elucidate underlying mechanisms contributing to cancer risk among obese individuals. Last, although obesity-related traits portend both increased risk for cancer and CVD in parallel, incidence rates of cancer are higher compared with those of CVD.
In prospective observational studies, BMI has been associated with incident cancers including gastrointestinal, colorectal, lung, gynecologic, and melanoma across broad populations.10,27, 28, 29 Furthermore, limiting weight gain in murine models,14 weight loss in patients with breast cancer,30 and weight loss after bariatric surgery in morbidly obese patients31 were associated with decreased tumor progression, recurrence, and cancer risk. Additionally, prior work from the FHS showed that abdominal adiposity as measured by WC was associated with obesity-related cancer including colorectal, breast, and female reproductive cancers.32,33 We now extend this prior work with additional follow-up across a larger sample. Although BMI was not associated with overall cancer, we observed higher risk for future gastrointestinal, colorectal, and gynecologic cancers and lower risk for future lung cancer, highlighting the importance of examining site-specific cancers. Our findings mirror those of a Swedish hospital registry–based prospective study that demonstrated significantly increased burden of overall cancer and site-specific cancer, including gastrointestinal and gynecologic, in obese compared with nonobese individuals13 and extend implications of obesity on cancer risk to a large community-based sample. Furthermore, we observed that greater abdominal adiposity (WC) was associated with increased risk for overall cancer, gastrointestinal, colorectal, and gynecologic cancers, which supports prior prospective epidemiologic studies.34,35
Notably, we found that greater BMI was associated with decreased risk for future lung cancer after adjustment for confounders including smoking status. This “obesity paradox” contrasts with other cancers in which greater BMI has been associated higher risk. This discrepancy has been demonstrated in numerous studies that suggest that higher BMI is associated with decreased risk for incident lung cancer and improved survival in patients with lung cancer.36,37 We explored this inverse association by stratifying lung cancer analyses by smoking status, as smoking is a known risk factor for lung cancer and is associated with lower average BMI.38 Our findings support a previous prospective cohort study of about 450,000 individuals,39 demonstrating reduced risk among current smoking but no association among former and never smoking. Although this might suggest that the “obesity paradox” may be explained by smoking, analyses of never smoking were likely underpowered, with other studies supporting an association across all smoking statuses including never smoking.28 We performed lag-time analyses starting 1 year after baseline examination to account for potentially undiagnosed cancers and found similar results. In contrast to the inverse association of BMI with lung cancer, both visceral and pericardial adipose tissue depots were associated with greater risk for lung cancer, as was inflammation assessed by CRP. This extends prior findings that abdominal adiposity appears to be associated with higher risk for lung cancer.36 Our findings highlight the importance of examining obesity-related phenotypes to better appreciate adiposity-associated cancer risk.
To provide insight into potential mechanisms linking obesity to increased predisposition to specific cancers, we further investigated the association of regional adipose depots with future cancer after accounting for BMI. We show that both visceral and pericardial adipose depots were associated with greater risk for overall cancer, lung cancer, and melanoma. In contrast, subcutaneous adipose depots were not associated with greater risk for future cancer. These findings are notable, as prior studies of VAT and cancer present conflicting findings.40, 41, 42, 43 The present study expands upon previous findings by assessing site-specific cancers in addition to overall cancer. In contrast to the “obesity paradox,” we show importantly and conversely that higher VAT was associated with increased risk for lung cancer, suggesting that BMI and obesity may not fully capture adiposity-associated risk. We also report that VAT was associated with melanoma, which builds upon previous studies showing an association of obesity with risk for melanoma and its progression.29,44 Previous retrospective studies have reported inverse associations of SAT with cancer mortality and progression of some cancer subtypes.45,46 Although we did not observe an association between SAT and overall cancer, we found that higher SAT was associated with protection against gynecologic cancer. To our knowledge, SAT has not previously been associated with cancer risk in a prospective inception cohort, though some studies have shown that increased SAT may portend better outcomes in patients with cancer.45,47
The biological mechanisms underlying greater cancer risk in obesity remain incompletely understood, but research has been focused on inflammation and immune cell dysfunction.7,48,49 It is known that VAT specifically appears to contribute to proinflammatory states,50,51 and our findings of associations of both WC and VAT with cancer risk support this hypothesis. In line with this, we examined circulating markers of systemic inflammation (CRP and PAI-1) previously associated with cancer52,53 and indeed found that CRP was significantly associated with overall cancer, colorectal, and lung cancer. These findings are in keeping with those of the recent CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study), in which baseline CRP was significantly higher in patients later diagnosed with lung cancer than those who did not develop lung cancer.54 Additionally, treatment with anti–interleukin-1β immunotherapy compared with placebo in CANTOS was associated with decreased lung cancer mortality and lung cancer incidence, further supporting the importance of inflammatory pathways as underlying mechanisms. In contrast, we did not find associations of PAI-1 with cancer risk.
Study limitations
First, this was an observational study, and we could not draw causal inferences or eliminate residual confounding. For example, surveillance bias may have led to greater cancer screening in obese vs nonobese individuals. This effect was likely limited given that survival after cancer diagnosis was not different between obese and nonobese individuals.
Second, our study may not be generalizable to the general population, as our sample was overwhelmingly White. Third, specific cancer subtype–stratified and sex-stratified analyses were exploratory, and results will need to be confirmed.
Finally, there were relatively small event numbers for specific cancer subtypes, which may have limited power to detect potentially modest but significant associations for secondary outcomes. For example, although some studies have shown differential sex patterns in obesity-associated cancers, including colorectal,55,56 our study did not reveal significant sex × BMI interactions for future cancers, as it may have been underpowered to detect such associations.
Our study’s strength, however, is the rigorous ascertainment of obesity and related adipose phenotypes across a harmonized dataset of 2 large inception cohorts with histologically confirmed cancer events, which offered the unprecedented opportunity to longitudinally examine the association of antecedent clinical factors with cancer risk in the community.
Conclusions
We report that obesity and abdominal adiposity (WC) were associated with increased future risk for cancer, including gastrointestinal, colorectal, and gynecologic cancers. Of note, although we found that obesity status and higher BMI were associated with lower risk for lung cancer, we further shed light onto these associations by specifically demonstrating that greater visceral and pericardial adipose tissue depots and circulating CRP were associated with greater risk overall cancer and site-specific cancers, including lung and melanoma. These findings highlight the importance of examining obesity-related phenotypes, including adipose tissue depots, to better understand the association between obesity and cancers. Although the association of obesity-related traits with CVD has been firmly established,57,58 our data now further support the notion that obesity is a shared risk factor between CVD and cancer.59 A novel aspect of this study is the examination of incident CVD and cancer side by side within the same cohort, with greater risk for both outcomes with higher body weight. In this context, it is notable that incidence rates of cancer outnumber CVD events, highlighting the importance of understanding long-term consequences of cardiometabolic disease with respect to both outcomes. Although initial studies suggest benefits of weight loss with respect to cancer-related outcomes, we acknowledge the multifaceted effects of weight loss and how it is achieved, which may vary. Future studies are needed to better understand mechanisms underlying obesity, abdominal adiposity, and cancer.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Obesity and abdominal adiposity as measured by WC are associated with increased risk for several specific cancers, including gastrointestinal and gynecologic cancer. Although BMI is conversely associated with lower risk for lung cancer, visceral adiposity confers higher lung cancer risk, highlighting the importance of examining obesity-related phenotypes.
TRANSLATIONAL OUTLOOK: Future studies are needed to elucidate the mechanisms underlying the association of obesity and obesity-related phenotypes with cancer.
Funding Support and Author Disclosures
The FHS was supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) (NO1-HC-25195, HHSN268201500001I, and 75N92019D00031). The PREVEND study was supported by a grant from the Dutch Kidney Foundation (E.033). Dr Ho was supported by grants from the National Institutes of Health (NIH) (R01-HL134893, R01-HL140224, and K24-HL153669). Drs Suthahar and de Boer are supported by grants from the Netherlands Heart Foundation (Netherlands CardioVascular Research Committee [CVON] SHE-PREDICTS-HF, grant 2017-21; CVON RED-CVD, grant 2017-11; CVON PREDICT2, grant 2018-30; and CVON DOUBLE DOSE, grant 2020B005) and by a grant from the European Research Council (Consolidator Grant 818715, SECRETE-HF). Dr Lau was supported by a grant from the NIH (5T32HL094301-07). Dr Cheng has been supported by the NHLBI (grants R01HL131532 and R01HL151828) and Zogenix. Dr Hussain has been supported by the National Cancer Institute (grant R01CA204145). Dr Benjamin was supported by the NIH/NHLBI (grants 2R01 HL092577, 2U54HL120163, and 1R01 HL141434), the NIH/National Institute on Aging (grant 1R01AG066010), the American Heart Association (grant AHA_18SFRN34110082), and the Robert Wood Johnson Foundation (grant 74624). Dr Vasan has been supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. Dr Ho has received research grants from Gilead Sciences and Bayer; and has received research supplies from EcoNugenics. The University Medical Center Groningen, which employs Drs De Boer and Suthahar, has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals, Ionis Pharmaceuticals, Novo Nordisk, and Roche. Dr de Boer has received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche. The views expressed in this paper are those of the authors and do not necessarily represent the view of the NHLBI, the NIH, or the U.S. Department of Health and Human Services. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental methods, tables, a figure, and references, please see the online version of this paper.
Appendix
References
- 1.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau E.S., Paniagua S.M., Liu E., et al. Cardiovascular risk factors are associated with future cancer. J Am Coll Cardiol CardioOnc. 2021;3:48–58. doi: 10.1016/j.jaccao.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung H., Siegel R.L., Torre L.A., et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112. doi: 10.3322/caac.21499. [DOI] [PubMed] [Google Scholar]
- 4.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity among adults and youth: United States, 2015-2016. NCHS Data Brief No. 288. https://www.cdc.gov/nchs/data/databriefs/db288.pdf Accessed February 9, 2022. [PubMed]
- 5.Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief No. 360. https://www.cdc.gov/nchs/data/databriefs/db360-h.pdf Accessed February 9, 2022. [PubMed]
- 6.Iyengar N.M., Gucalp A., Dannenberg A.J., Hudis C.A. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. doi: 10.1200/JCO.2016.67.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb R., Sutterwala F.S., Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77–89. doi: 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijers W.C., de Boer R.A. Common risk factors for heart failure and cancer. Cardiovasc Res. 2019;115:844–853. doi: 10.1093/cvr/cvz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ringel A.E., Drijvers J.M., Baker G.J., et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell. 2020;183:1848–1866.e26. doi: 10.1016/j.cell.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy N., Jenab M., Gunter M.J. Adiposity and gastrointestinal cancers: epidemiology, mechanisms and future directions. Nat Rev Gastroenterol Hepatol. 2018;15:659–670. doi: 10.1038/s41575-018-0038-1. [DOI] [PubMed] [Google Scholar]
- 11.Sung H., Siegel R.L., Rosenberg P.S., Jemal A. Emerging cancer trends among young adults in the USA: analysis of a population-based cancer registry. Lancet Public Health. 2019;4:e137–e147. doi: 10.1016/S2468-2667(18)30267-6. [DOI] [PubMed] [Google Scholar]
- 12.Steele C.B., Thomas C.C., Henley S.J., et al. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2017;66:1052–1058. doi: 10.15585/mmwr.mm6639e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolk A., Gridley G., Svensson M., et al. A prospective study of obesity and cancer risk (Sweden) Cancer Causes Control. 2001;12:13–21. doi: 10.1023/a:1008995217664. [DOI] [PubMed] [Google Scholar]
- 14.Lauby-Secretan B., Scoccianti C., Loomis D., et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention Body mass index: considerations for practitioners. https://www.cdc.gov/obesity/downloads/bmiforpactitioners.pdf Accessed April 13, 2021.
- 16.Zhu S., Wang Z., Heshka S., Heo M., Faith M.S., Heymsfield S.B. Waist circumference and obesity-associated risk factors among Whites in the third National Health and Nutrition Examination Survey: clinical action thresholds. Am J Clin Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 17.Lee K.R., Seo M.H., Do Han K., Jung J., Hwang I.C. for the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Waist circumference and risk of 23 site-specific cancers: a population-based cohort study of Korean adults. Br J Cancer. 2018;119:1018–1027. doi: 10.1038/s41416-018-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawber T.R., Meadors G.F., Moore F.E. Epidemiological approaches to heart disease: the Framingham study. Am J Public Health Nations Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kannel W.B., Feinleib M., McNamara P.M., Garrison R.J., Castelli W.P. An investigation of coronary heart disease in families: the Framingham Offspring Heart Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 20.Splansky G.L., Corey D., Yang Q., et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 21.Pinto-Sietsma S.J., Janssen W.M.T., Hillege H.L., Navis G., Zeeuw D.D., Jong P.E.D. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000;11:1882–1888. doi: 10.1681/ASN.V11101882. [DOI] [PubMed] [Google Scholar]
- 22.Fox C.S., Massaro J.M., Hoffmann U., et al. Abdominal visceral and subcutaneous adipose tissue compartments association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 23.Rosito G.A., Massaro J.M., Hoffmann U., et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 24.Maurovich-Horvat P., Massaro J., Fox C.S., Moselewski F., O’Donnell C.J., Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes. 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 25.Kreger B.E., Splansky G.L., Schatzkin A. The cancer experience in the Framingham Heart Study cohort. Cancer. 1991;67:1–6. doi: 10.1002/1097-0142(19910101)67:1<1::aid-cncr2820670102>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 26.Casparie M., Tiebosch A.T.M.G., Burger G., et al. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol. 2007;29:19–24. doi: 10.1155/2007/971816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colditz G.A., Peterson L.L. Obesity and cancer: evidence, impact, and future directions. Clin Chem. 2018;64:154–162. doi: 10.1373/clinchem.2017.277376. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Dong J., Sun K., et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132:1162–1169. doi: 10.1002/ijc.27719. [DOI] [PubMed] [Google Scholar]
- 29.Clement E., Lazar I., Muller C., Nieto L. Obesity and melanoma: could fat be fueling malignancy? Pigment Cell Melanoma Res. 2017;30:294–306. doi: 10.1111/pcmr.12584. [DOI] [PubMed] [Google Scholar]
- 30.Chlebowski R.T., Blackburn G.L., Thomson C.A., et al. Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins T., Antonowicz S.S., Markar S.R. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg. 2019;29:1031–1039. doi: 10.1007/s11695-018-3501-8. [DOI] [PubMed] [Google Scholar]
- 32.Moore L.L., Bradlee M.L., Singer M.R., et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham study adults. Int J Obes Relat Metab Disord. 2004;28:559–567. doi: 10.1038/sj.ijo.0802606. [DOI] [PubMed] [Google Scholar]
- 33.Moore L.L., Chadid S., Singer M.R., Kreger B.E., Denis G.V. Metabolic health reduces risk of obesity-related cancer in Framingham study adults. Cancer Epidemiol Biomarkers Prev. 2014;23:2057–2065. doi: 10.1158/1055-9965.EPI-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barberio A.M., Alareeki A., Viner B., et al. Central body fatness is a stronger predictor of cancer risk than overall body size. Nat Commun. 2019;10 doi: 10.1038/s41467-018-08159-w. 383-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White A.J., Nichols H.B., Bradshaw P.T., Sandler D.P. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121:3700–3708. doi: 10.1002/cncr.29552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D., Zheng W., Johansson M., et al. Overall and central obesity and risk of lung cancer: a pooled analysis. J Natl Cancer Inst. 2018;110:831–842. doi: 10.1093/jnci/djx286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam V.K., Bentzen S.M., Mohindra P., et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC) Lung Cancer. 2017;104:52–57. doi: 10.1016/j.lungcan.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Audrain-McGovern J., Benowitz N. Cigarette smoking, nicotine, and body weight. Clin Pharmacol Ther. 2011;90:164–168. doi: 10.1038/clpt.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith L., Brinton L.A., Spitz M.R., et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104:778–789. doi: 10.1093/jnci/djs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto S., Nakagawa T., Matsushita Y., et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care. 2010;33:184–189. doi: 10.2337/dc09-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erarslan E., Turkay C., Koktener A., Koca C., Uz B., Bavbek N. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci. 2009;54:862–868. doi: 10.1007/s10620-008-0440-6. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Perez S.L., Chaudhry V., Mar W., et al. Impact of abdominal adipose depots and race on risk for colorectal cancer: a case-control study. Nutr Cancer. 2017;69:573–579. doi: 10.1080/01635581.2017.1296964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Britton K.A., Massaro J.M., Murabito J.M., Kreger B.E., Hoffmann U., Fox C.S. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. J Am Coll Cardiol. 2013;62:921–925. doi: 10.1016/j.jacc.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandon E.L., Gu J.-W., Cantwell L., He Z., Wallace G., Hall J.E. Obesity promotes melanoma tumor growth: role of leptin. Cancer Biol Ther. 2009;8:1871–1879. doi: 10.4161/cbt.8.19.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ebadi M., Martin L., Ghosh S., et al. Subcutaneous adiposity is an independent predictor of mortality in cancer patients. Br J Cancer. 2017;117:148–155. doi: 10.1038/bjc.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greco F., Mallio C., Cirimele V., Grasso R., Zobel B. Subcutaneous adipose tissue as a biomarker of pancreatic cancer: a pilot study in male patients. Clin Cancer Investig J. 2019;8:114–118. [Google Scholar]
- 47.Charette N., Vandeputte C., Ameye L., et al. Prognostic value of adipose tissue and muscle mass in advanced colorectal cancer: a post hoc analysis of two non-randomized phase II trials. BMC Cancer. 2019;19 doi: 10.1186/s12885-019-5319-8. 134-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karimi K., Lindgren T.H., Koch C.A., Brodell R.T. Obesity as a risk factor for malignant melanoma and non-melanoma skin cancer. Rev Endocr Metab Disord. 2016;17:389–403. doi: 10.1007/s11154-016-9393-9. [DOI] [PubMed] [Google Scholar]
- 49.Berger N.A. Young adult cancer: influence of the obesity pandemic. Obesity (Silver Spring) 2018;26:641–650. doi: 10.1002/oby.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maury E., Ehala-Aleksejev K., Guiot Y., Detry R., Vandenhooft A., Brichard S.M. Adipokines oversecreted by omental adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2007;293:E656–665. doi: 10.1152/ajpendo.00127.2007. [DOI] [PubMed] [Google Scholar]
- 51.Yu J.Y., Choi W.J., Lee H.S., Lee J.W. Relationship between inflammatory markers and visceral obesity in obese and overweight Korean adults: an observational study. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000014740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S., Choe J.W., Kim H.K., Sung J. High-sensitivity C-reactive protein and cancer. J Epidemiol. 2011;21:161–168. doi: 10.2188/jea.JE20100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S., Wei X., He J., Tian X., Yuan S., Sun L. Plasminogen activator inhibitor-1 in cancer research. Biomed Pharmacother. 2018;105:83–94. doi: 10.1016/j.biopha.2018.05.119. [DOI] [PubMed] [Google Scholar]
- 54.Ridker P.M., MacFadyen J.G., Thuren T., et al. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 55.Kim H.I., Lim H., Moon A. Sex differences in cancer: epidemiology, genetics and therapy. Biomol Ther (Seoul) 2018;26:335–342. doi: 10.4062/biomolther.2018.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim H., Giovannucci E.L. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28:1–4. doi: 10.1007/s10552-016-0831-5. [DOI] [PubMed] [Google Scholar]
- 57.D’Agostino R.B., Vasan R.S., Pencina M.J., et al. General cardiovascular risk profile for use in primary care. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 58.Fox C.S., Pencina M.J., Wilson P.W.F., Paynter N.P., Vasan R.S., D’Agostino R.B. Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham Heart Study. Diabetes Care. 2008;31:1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson C.B., Davis M.K., Law A., Sulpher J. Shared risk factors for cardiovascular disease and cancer: implications for preventive health and clinical care in oncology patients. Can J Cardiol. 2016;32:900–907. doi: 10.1016/j.cjca.2016.04.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.