Central Illustration
Cumulative anthracycline doses can cause heart failure (HF). Cancer therapy regimens now typically adopt lower doses than in the past, but subclinical cardiotoxicity that potentially progresses toward HF may occur, particularly if patients have comorbidities. We previously reported that comorbidity-free patients had a risk for asymptomatic diastolic dysfunction (DD) 1 week after completing anthracycline-based regimens.1 Here we aimed to define DD risk versus HF risk using both a preclinical model and human data.
Our analysis included 60 patients (50 women), 49 ± 9 years of age, treated with doxorubicin (n = 18) or epirubicin (n = 25) for early breast cancer (primarily anthracycline-cyclophosphamide-taxane regimens) or treated with a rituximab-cyclophosphamide-doxorubicin-vincristine-prednisone regimen for newly diagnosed non-Hodgkin lymphoma (n = 17).1 The study was approved by the Institutional Review Boards of participating centers, and written informed consent was obtained from all patients. The mean cumulative doxorubicin-equivalent dose was 258 ± 34 mg/m2 (epirubicin dose converted by a doxorubicin equivalence ratio of 0.66).2 After chemotherapy, left ventricular ejection fraction was unchanged from baseline (61% ± 4% vs. 62% ± 5%; P = 0.47), but incident grade I DD was diagnosed in 13 patients (22% incidence).
To establish the relationship between cardiotoxicity risk and anthracycline dose, we previously correlated HF risk with anthracycline infusions and cardiac anthracycline accumulation in a preclinical model.2 Human myocardial samples were exposed to simulations of single infusions of 60 mg doxorubicin/m2 or an equivalent 90 mg epirubicin/m2. The size of postinfusion cardiac anthracycline pools was determined, and HF risk from repeat infusions was estimated as follows:
| (1) |
where pooln is the size of the cardiac anthracycline pool after n infusions, risk pool is the cardiac anthracycline pool associated with 5% HF risk (31 nmol/g for either anthracycline), and c is a correction constant for the different cardiotoxic potential of doxorubicin or epirubicin.2 The model predicted 5% risk for HF after 380 mg doxorubicin/m2 or 607 mg epirubicin-based doxorubicin equivalent/m2, in excellent agreement with reported values of 400 or 594 mg/m2 in comorbidity-free patients, respectively.2
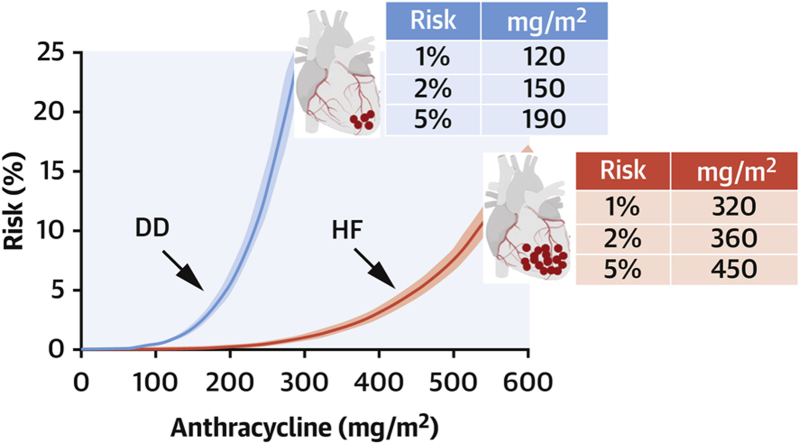
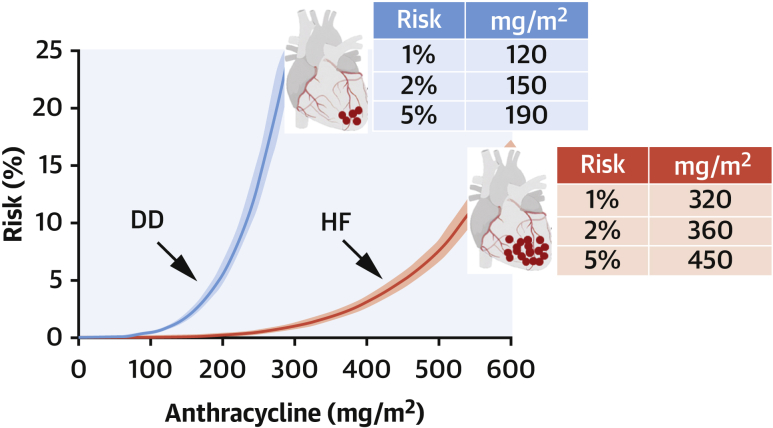
As it pertains to DD, given that the risk pool was unknown, the risk was assumed to be 22%, and a mean risk pool of 17 nmol/g was calculated from infusion doses and the total number of infusions in our study population,1 and equation 1 was solved accordingly. For patients receiving infusion doses other than 60 or 90 mg/m2 doxorubicin or epirubicin, the size of postinfusion anthracycline pools was adjusted proportionally. Exponential risk-versus-dose curves were obtained that identified 1%, 2%, and 5% DD risk after 120, 150, and 190 mg anthracycline/m2. For HF, the corresponding doses for 1%, 2%, and 5% risk were 320, 360, and 450 mg/m2 (Figure 1). Thus, DD risk occurred at significantly lower anthracycline doses than HF (P ≤ 0.006 by receiver-operating characteristic curve analysis).
Figure 1.
Risk-Versus-Dose Relationship for Anthracycline-Related Diastolic Dysfunction and Heart Failure
Curves (means with 95% confidence intervals) were obtained using equation 1, correlating risk with cardiac anthracycline accumulation, as described in the text. Diastolic dysfunction (DD) occurred after lower cumulative doses and less anthracycline accumulation than heart failure (HF).
There are limitations to this study. Diastolic function was evaluated using transmitral flow indexes such as E/A ratio and deceleration time of the E wave. Grade I DD (impaired relaxation) was diagnosed by E/A decrement toward or below the lower limit of normal and concomitant DT prolongation; however, recommendations for diastolic function evaluation have changed since our clinical study was conducted. Other indexes are now recommended to diagnose and grade DD. Information using mitral E/e′ ratio is recommended, but we note that this parameter may be less useful in patients without HF symptoms and left ventricular ejection fraction >50%, as was the case with our study population.3 Accordingly, in our analysis, when E/e′ was measured at the investigator’s discretion, this was normal in patients diagnosed with grade I DD by E/A and DT.1 Moreover, many factors were taken into consideration to mitigate bias: 1) all patients were assessed for diastolic function 1 week after the last chemotherapy cycle, when they had recovered from treatment-related fluid overload; 2) E/A and DT were normalized to age-related ranges of normality (1 week vs. baseline: −2% ± 8% vs. 41% ± 29% for E/A, 93% ± 20% vs. 39% ± 27%; P < 0.001); 3) each center designated a study-dedicated operator to minimize interobserver variability, and all echocardiograms were centrally reviewed by a blinded operator; 4) patients with or without DD had similar distributions of cardiovascular parameters that could alter E/A and DT (systolic blood pressure [P = 0.39], diastolic blood pressure [P = 0.99], heart rate [P = 0.81], and body mass index [P = 0.94]).
Our study also had strengths and suggests potential clinical implications. By demonstrating that 120 mg anthracycline/m2 was sufficient to cause a 1% risk for early manifestation of DD in comorbidity-free patients, our findings support the notion that cardiotoxicity begins with the first anthracycline infusion, and hence there is no safe anthracycline dose.4 Correlations between cardiac anthracycline accumulation and risk for cardiotoxicity call for a wider exploitation of slow infusions or liposomal formulations that diminish cardiac exposure to and accumulation of anthracyclines.4 Alternatively, one might consider dexrazoxane, which does not diminish cardiac anthracycline accumulation but prevents cardiotoxicity by reducing formation of DNA-damaging anthracycline-topoisomerase β2 complexes in cardiomyocytes.4 Cancer survivors should also be given an adequate cardiac surveillance.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Calabrese V., Menna P., Annibali O., et al. Early diastolic dysfunction after cancer chemotherapy: primary endpoint results of a multicenter cardio-oncology study. Chemotherapy. 2018;63:55–63. doi: 10.1159/000486761. [DOI] [PubMed] [Google Scholar]
- 2.Salvatorelli E., Menna P., Chello M., et al. Low-dose anthracycline and risk of heart failure in a pharmacokinetic model of human myocardium exposure: analog specificity and role of secondary alcohol metabolites. J Pharmacol Exp Ther. 2018;364:323–331. doi: 10.1124/jpet.117.246140. [DOI] [PubMed] [Google Scholar]
- 3.Previtali M., Chieffo E., Ferrario M., Klersy C. Is mitral E/E′ ratio a reliable predictor of left ventricular diastolic pressures in patients without heart failure? Eur Heart J Cardiovasc Imaging. 2012;13:588–595. doi: 10.1093/ejechocard/jer286. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin R.S., Minotti G. Doxorubicin-dexrazoxane from day 1 for soft-tissue sarcomas: the road to cardioprotection. Clin Cancer Res. 2021;27:3809–3811. doi: 10.1158/1078-0432.CCR-21-1376. [DOI] [PubMed] [Google Scholar]