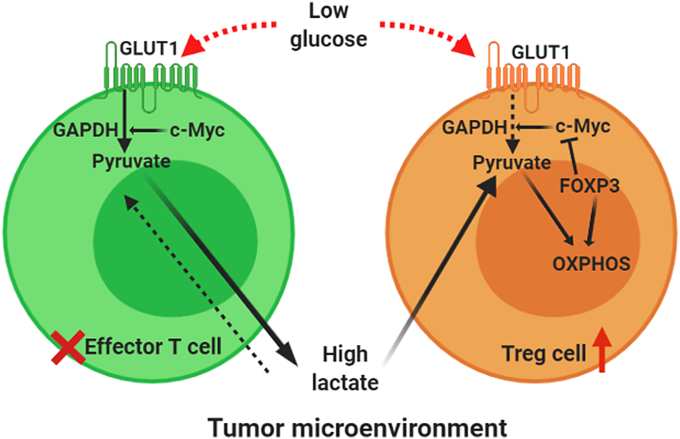
Fig. 3.
Overview of metabolism of effector T cells and the reprogrammed metabolism of tumor-infiltrating Tregs: Under normal condition, activated effector T cells highly express GLUT1 (glucose transporter) and c-Myc which supports the glycolytic pathway through which these effector cells survive in normal conditions. In TME this dependency on glycolysis becomes fatal for effector T cells as in the high lactate low glucose tumor microenvironment, they face a redox imbalance and thus a key regulatory enzyme of glycolysis, GAPDH can not function. Whereas in Tregs FOXP3 blocks the expression of c-Myc and this can be one possible mechanism of this metabolic shift from glucose to fatty acid dependency. Tumor-infiltrating Tregs oxidize NADH to NAD+ through the coupled action of ETC and TCA cycle. And as a result, the effector T cells which are dependent on glucose for their growth and maintenance cannot survive in low glucose lactate rich tumor microenvironment. (Created in BioRender.com).