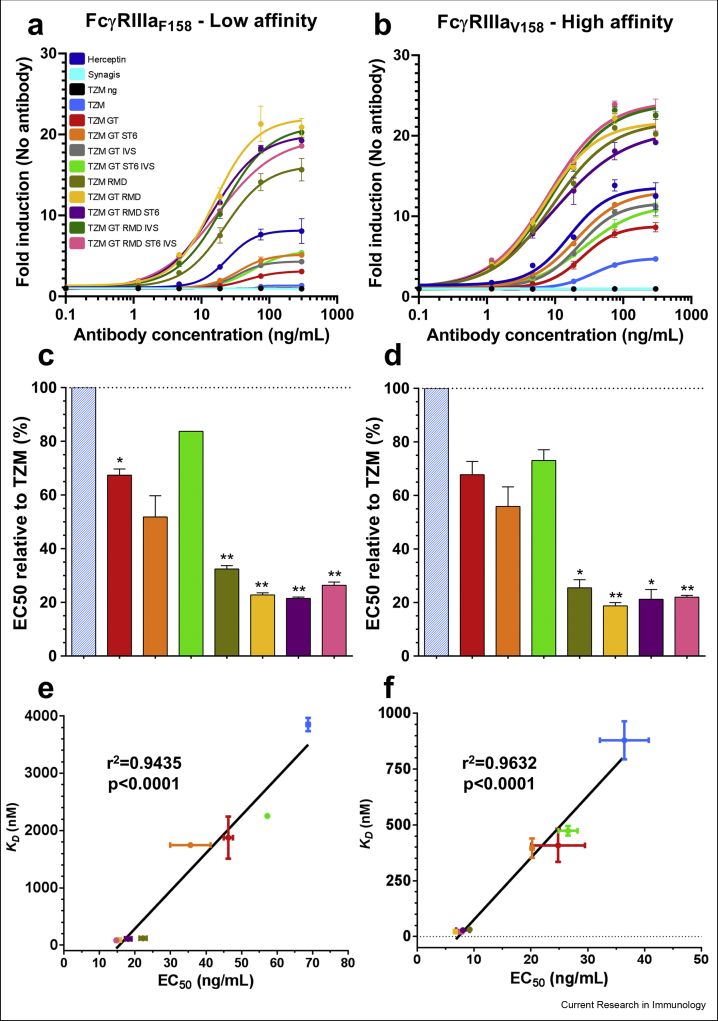
Fig. 7.
Reporter assay responses induced by the TZM glycoforms. The antibodies were put in contact with target cells expressing HER2 and effector cells expressing the low affinity FcγRIIIaF158 (a) or the high affinity FcγRIIIaV158 (b). The response was measured as a bioluminescent signal produced by the effector cells upon antibody binding, and expressed as a multiple of the basal bioluminescent signal produced by the effector cells in absence of antibodies. The results are the average and standard deviations of duplicates. Commercial Herceptin was included in the assays as positive control while Synagis and non-glycosylated TZM (TZM ng) were included as negative controls. The half maximal effective concentration (EC50) for each TZM glycoforms was calculated using a non-linear fit (least squares) with a variable slope and displayed as relative EC50 compared to TZM (hatched columns) for the FcγRIIIaF158 (c) and FcγRIIIaV158 (d). The data represent the mean and SD; ∗ and ∗∗ denote a statistical significance of p ≤ 0.05 and p ≤ 0.01, respectively, as tested by a one-sample t-test against a theoretical mean of 100 (%). Correlation between apparent KD values of FcγRIIIaF158 (e) or FcγRIIIaV158 (f) binding of TZM glycoforms and EC50. r2 and p value of the linear regression are shown.