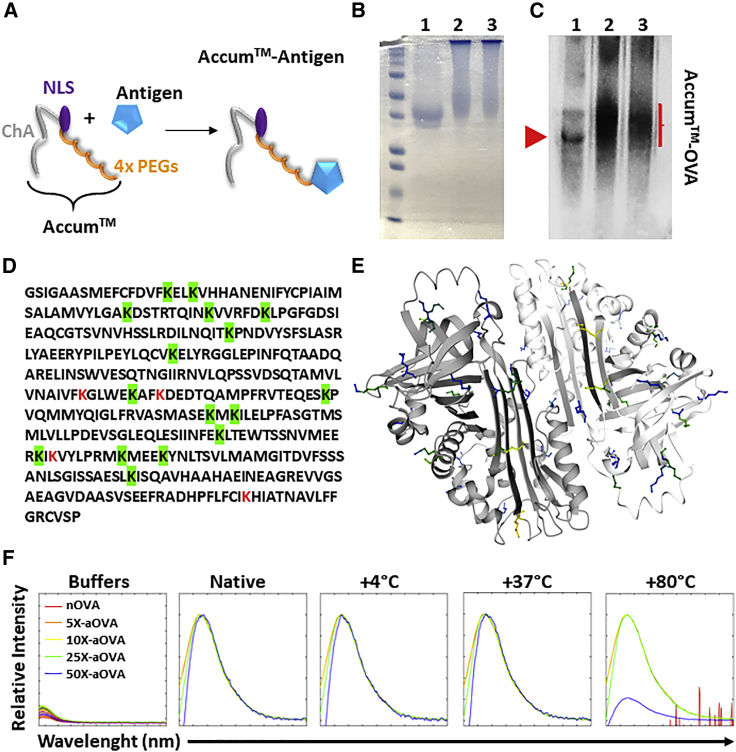
Figure 1.
Biochemical characterization of the Accum-antigen formulation
(A) Schematic diagram representing covalent binding of a given antigen to the Accum moiety (ChA, NLS and 4x PEGs).
(B) A representative Coomassie blue staining displaying OVA (line 1), aOVA at a ratio of 25X (line 2), and aOVA at a ratio of 50X (line 3).
(C) A representative western blot of the gel shown in (B).
(D) The amino acid sequence of chicken OVA. Lysine residues that are predicted to be accessible for Accum linking (>50%) are highlighted in green. The three weakly accessible residues are shown in red.
(E) A ribbon structure of the OVA protein with lysine residues that are predicted to be highly (in blue), moderately (green), or poorly (yellow) accessible lysine residues.
(F) ITF analysis of nOVA or aOVA at various Accum to OVA ratios in response to thermal stress. The experiment presented in (B) was repeated at least 10 times, whereas (F) is a representative study of two independent repeats.