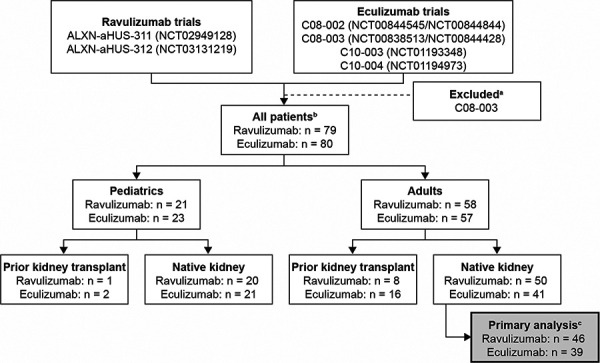
Figure 1. Flow of patients included in the primary analysis dataset. aData from eculizumab trial C08-003 were excluded owing to the study being conducted in a different patient population (patients were receiving long-term maintenance plasma therapy at baseline and, consequently, had normal platelet counts). b“All patients” represents the maximum possible number of patients before the application of missing data restrictions for each subgroup by treatment. cPatient numbers for primary analysis represent patients with complete cases for propensity score variables, with a maximum of one missing laboratory measure and outcome data within 56 days of the 6-month endpoint.