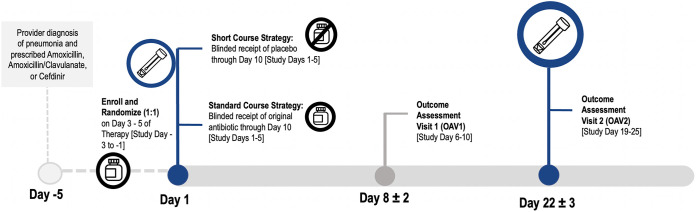
FIG 1.
SCOUT-CAP study design and timeline. SCOUT-CAP was a multicenter, randomized, double-blind, placebo-controlled, superiority clinical trial, which evaluated a short course (5 days) versus standard course (10 days) strategy of beta-lactam therapy for outpatient treatment of pediatric CAP. Participants were enrolled on days 3 to 6 of their initially prescribed oral beta-lactam therapy and randomized to 5 days of matching placebo (short course strategy) or an additional 5 days of their prestudy antibiotic (standard course strategy). Outcome assessment visits (OAV) occurred on study day 6 to 10 (OAV1) and study day 19 to 25 (OAV2). Throat swabs used in this study were collected at enrollment and OAV2.