Figure 1. Timeline of key activities and licensure changes for Anthrax Vaccine Adsorbed from 1955–2015.
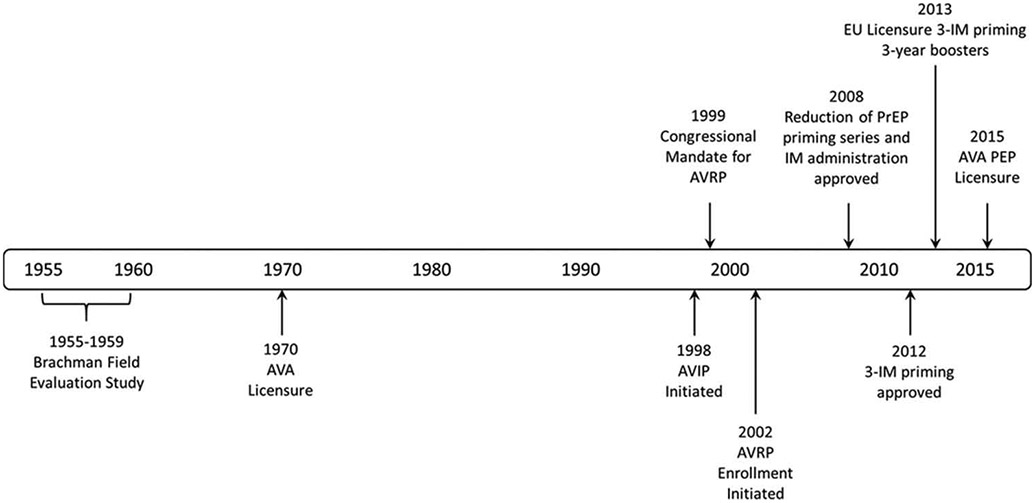
1955–1959 – Field evaluation of a predecessor anthrax vaccine by Brachman et al. [60]. Vaccine effectiveness to prevent both inhalation and cutaneous anthrax combined was 92.5% (lower 95%CI, 65%).
1962–1974 – CDC surveillance data on the occurrence of anthrax disease in at-risk industrial settings. Data were supportive of the effectiveness of AVA and an earlier version of anthrax vaccine. No cases of anthrax occurred in fully vaccinated subjects although the risk of infection continued (http://www.gpo.gov/fdsys/pkg/FR-2004-12-29/html/04-28322.htm).
1998 – Initiation of the DoD Anthrax Vaccine Immunization Program (AVIP), for mandatory vaccination of service members.
1999 – Announcement of congressional mandate for the CDC Anthrax Vaccine Research Program (AVRP).
2002 – Enrollment in the AVRP initiated.
2008 – AVRP interim analysis; FDA approved change of route of AVA administration to IM and reduction of PrEP priming series to 5 doses over 18 months, with annual boosters.
2012 – AVRP final analysis; FDA approved change to modify PrEP priming series to 3 IM doses over 6 months, with boosters at 12 and 18 months, and annually thereafter.
2013 – First approval to market AVA in the European Union under a 3-IM priming series and 3-yearly booster schedule (http://bioprepwatch.com/stories/510510714-emergent-biosolutions-receives-approval-to-market-anthrax-vaccine-in-germany).
2015 – AVA approved for PEP indication; first use of the ‘animal rule’ for licensure of a vaccine (http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf
