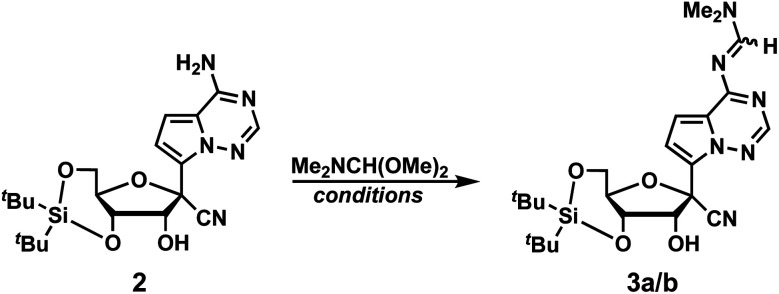
Optimization of exocyclic amine 2 protection using N,N-dimethylformamide dimethyl acetala.
| ||||
|---|---|---|---|---|
| Entry | Temp (°C) | Time (h) | Solvent | Yielde (%) |
| 1 | RT | 48 | DMF | 66 |
| 2 | 65 | 24 | DMF | 58 |
| 3b | 65 | 24 | DMF | 57 |
| 4c | 65 | 24 | DMF | 55 |
| 5d | 65 | 1 | DMF | 59 |
| 6 | 65 | 24 | DMA | 52 |
| 7 | 65 | 24 | Pyridine | 57 |
| 8f | 65 | 24 | — | 71 |
Unless otherwise stated, the reactions were performed on a 0.10 mmol scale using 2 (1.0 equiv.) and N,N-dimethylformamide dimethyl acetal (4.0 equiv.) in the corresponding solvent (1.0 mL organic solvent).
Reaction performed with MS 4 Å (50 mg).
Reaction performed with MgSO4 (50 mg).
Reaction was performed in a microwave synthesizer at 200 W, 17 PSI.
Yield represents a mixture of diastereomers (for entries 1–7 the reaction resulted in 3a as the major diastereomer and 3b as the minor diastereomer).
Reaction yielded 3b as the only diastereomer. DMF: N,N-dimethyl formamide; DMA: N,N-dimethyl formamide acetal. Reactions were performed in duplicate and yields shown are an average. Note: 3a and 3b are separable diastereomers; stereochemical assignments of the imines of 3a and 3b were not conducted since both protecting groups are ultimately cleaved.
