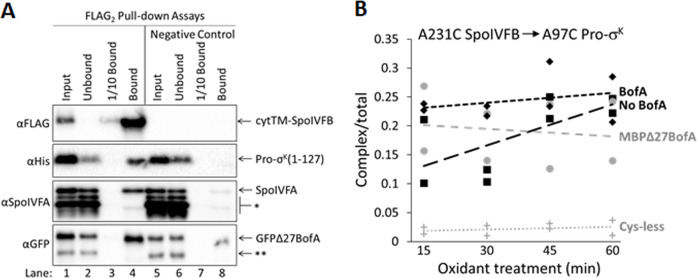
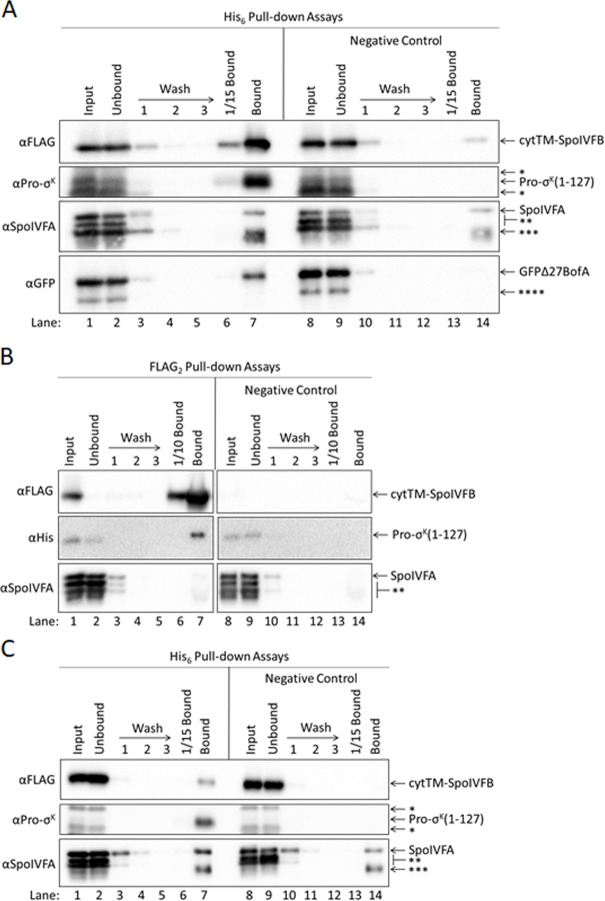
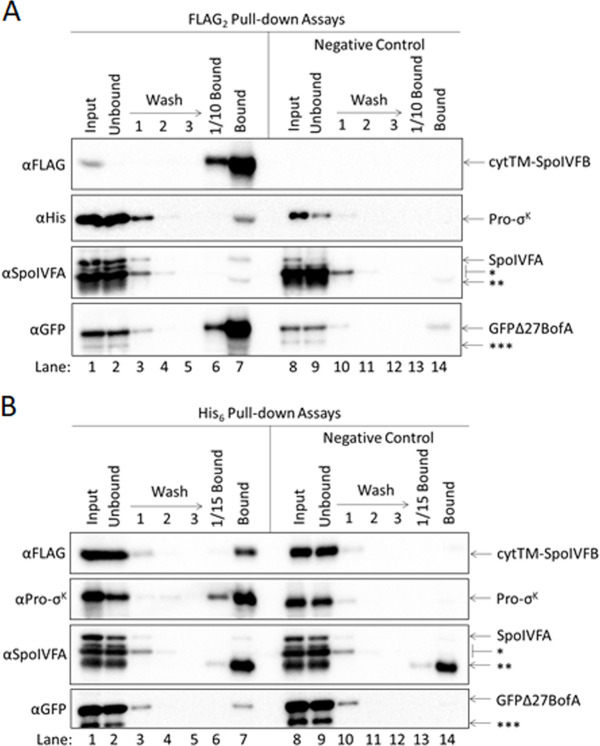
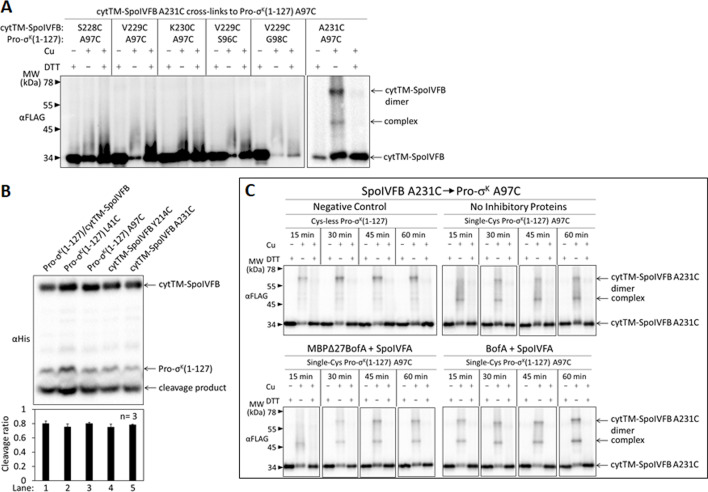
Figure 5. Inhibitory proteins do not prevent Pro-σK(1–127) from interacting with SpoIVFB.
(A) Pro-σK(1–127), SpoIVFA, and GFPΔ27BofA co-purify with cytTM-SpoIVFB. pET Quartet plasmids were used to produce a catalytically inactive E44C cytTM-SpoIVFB variant with a FLAG2 tag (pSO73), or a variant lacking FLAG2 as a negative control (pSO149), in combination with Pro-σK(1–127), SpoIVFA, and GFPΔ27BofA in Escherichia coli. Samples collected after 2 hr of IPTG induction were subjected to co-immunoprecipitation with anti-FLAG antibody beads. Input, unbound, 1/10 bound (diluted to match input), and (undiluted) bound samples were subjected to immunoblot analysis with FLAG, penta-His, SpoIVFA, and GFP antibodies. The single star (*) indicates cross-reacting proteins below SpoIVFA and the double star (**) indicates a cross-reacting protein or breakdown species of GFPΔ27BofA that fail to co-purify. A representative result from two biological replicates is shown. (B) A231C in the cytTM-SpoIVFB CBS domain forms disulfide cross-links with A97C in the C-terminal region of Pro-σK(1–127) in the absence or presence of inhibitory proteins. pET Duet plasmids were used to produce single-Cys A231C cytTM-SpoIVFB E44Q in combination with single-Cys A97C Pro-σK(1–127) (pSO130; squares and line with long dashes labeled ‘No BofA’, although SpoIVFA is also absent) or with Cys-less Pro-σK(1–127) as a negative control (pSO255; crosses and line labeled ‘Cys-less’) in E. coli. pET Quartet plasmids were used to produce single-Cys A231C cytTM-SpoIVFB E44Q, single-Cys A97C Pro-σK(1–127), and Cys-less SpoIVFA in combination with Cys-less MBPΔ27BofA (pSO133; circles and line labeled ‘MBPΔ27BofA’) or with Cys-less full-length BofA (pSO246; diamonds and line labeled ‘BofA’) in E. coli. Samples collected after 2 hr of IPTG induction were treated for 15, 30, 45, or 60 min with Cu2+(phenanthroline)3 oxidant to promote disulfide bond formation and subjected to immunoblot analysis with FLAG antibodies to visualize the cytTM-SpoIVFB monomer, dimer, and complex with Pro-σK(1–127) (Figure 5—figure supplement 3C). Abundance of the complex was divided by the total amount of cytTM-SpoIVFB monomer, dimer, and complex. The ratio over time was plotted (n=2) with a best-fit trend line.