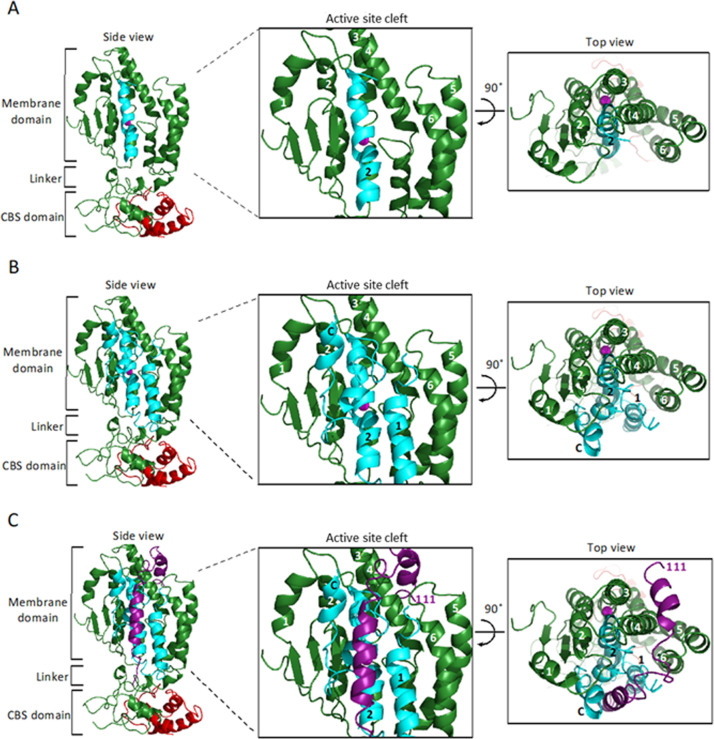
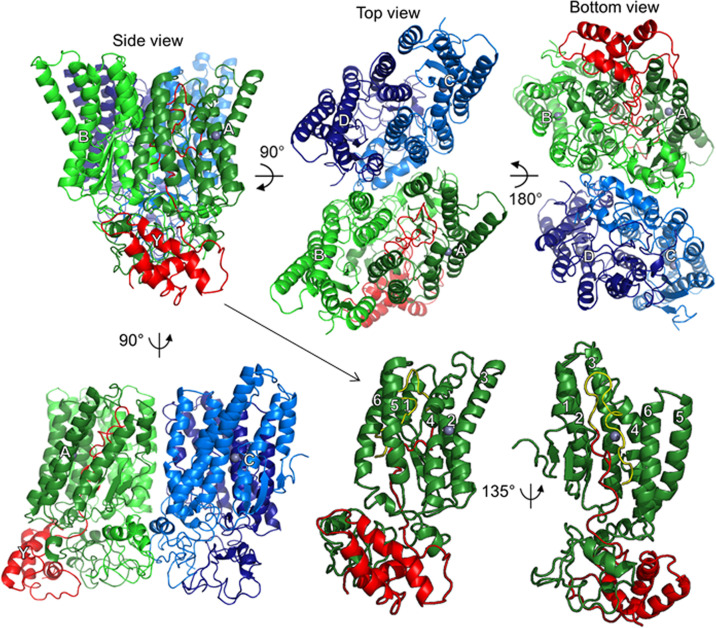
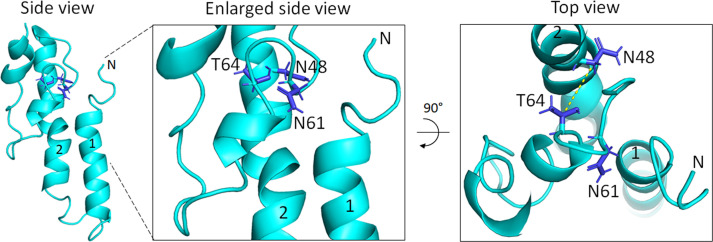
Figure 7. Model of SpoIVFB monomer with BofA and parts of SpoIVFA and Pro-σK.
(A) Model of SpoIVFB, BofA TMS2, and the C-terminal part of Pro-σK(1–127). At Left, a side view of the complex, showing the six TMSs of the SpoIVFB membrane domain with the active site zinc ion (magenta), the interdomain linker, and the CBS domain (green), BofA TMS2 (cyan), and Pro-σK(38–114) (red). In the enlarged view of the active site cleft (Center), TMSs 1–6 of SpoIVFB and TMS2 of BofA are numbered. At Right, a top view is shown. (B) Model of SpoIVFB with full-length BofA and Pro-σK(38–114). Predicted TMSs 1 and 2 of BofA are numbered and its C-terminal region is labeled ‘C’ near the C-terminus in the views shown in the Center and at Right. (C) Model of SpoIVFB with full-length BofA, SpoIVFA(65–111) (purple, residue 111 is numbered), and Pro-σK(38–114). TMS, transmembrane segment.