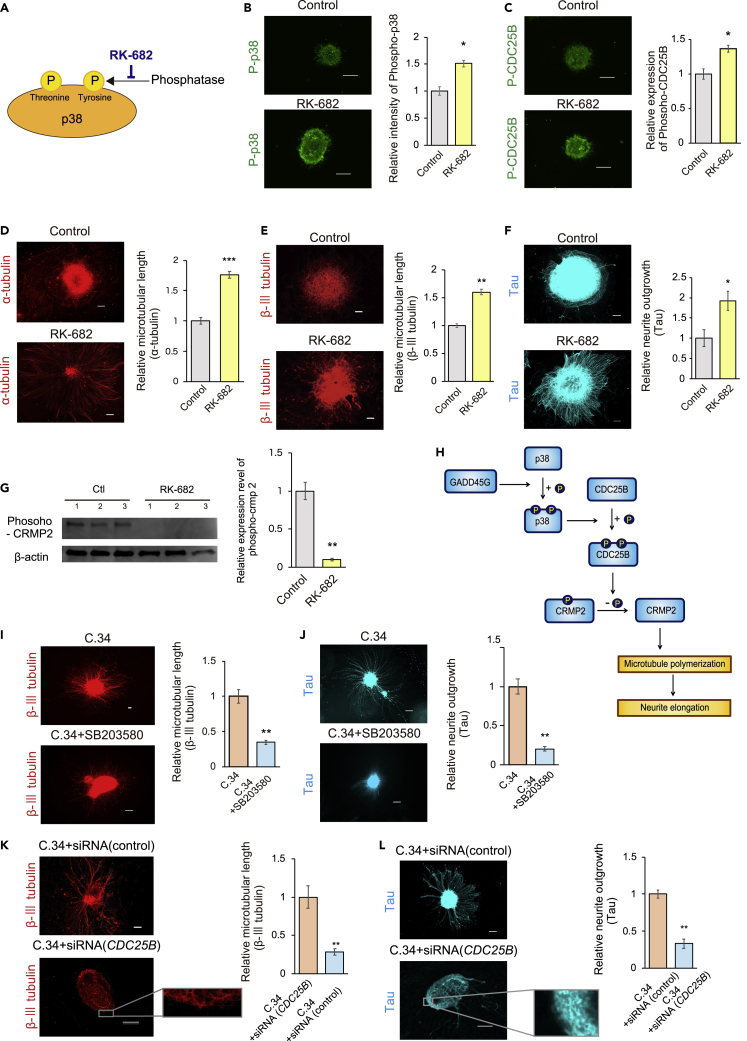
Figure 4.
GADD45G/p38 MAPK/CDC25B signaling dephosphorylates phospho-CRMP2 and elongates neurites
(A) Conceptual diagram depicting the inhibition of p38 tyrosine dephosphorylation by RK-682.
(B) Immunostaining images and a quantitative graph showing that neurospheres treated with RK-682 expressed phospho-p38 at higher levels than those not treated with RK-682 (n = 3, p = 0.0319).
(C) Immunostaining images and quantitative graph showing that neurospheres treated with RK-682 expressed phospho-CDC25B at higher levels than those not treated with RK-682 (n = 3; p = 0.0162).
(D) Immunostaining images of α-tubulin and a quantitative graph of the microtubule length. The length was greater in the RK-682-treated group than in the untreated group (n = 3; p = 0.000635).
(E) Immunostaining images of β-III tubulin and a quantitative graph of the microtubule length. The length was greater in the RK-682-treated group than in the untreated group.
(F) Immunostaining images of tau and a quantitative graph of neurites. Neurites were longer in the RK-682-treated group than in the untreated group (n = 3; p = 0.0434).
(G) The expression level of phospho-CRMP2 was quantified by western blotting in neurons supplemented with RK-682 and neurons not supplemented with RK-682.
(H) Conceptual diagram of the signaling pathway that causes neurite outgrowth. Starting with GADD45G, dephosphorylation of CRMP2 occurs via phosphorylation of p38 MAPK and CDC25B, which promotes the polymerization of microtubules and causes neurite outgrowth.
(I and J) Neurospheres treated with only Compound 34 and with both Compound 34 and SB203580 were cultured for 14 days. Differentiated neurons were immunostained with β-III tubulin (I) or tau (J). The lengths of microtubules and neurites were compared between the SB203580-treated group and the untreated group. The extension of neurites was suppressed in the SB203580 group (n = 3; p = 0.00270, p = 0.00122).
(K and L) The lengths of microtubules and neurites were not extended by the siRNA-mediated knockdown of CDC25B. After neuronal differentiation, immunostaining of β-III tubulin (K) or tau (L) was performed. Neuronal differentiation was not inhibited even in the CDC25B knockdown group (high-magnification image). Neurite elongation was inhibited in the CDC25B knockdown group (n = 3; p = 0.00994, p = 0.00286).Statistical analyses were performed with unpaired two-tailed Student’s t-tests. The values in the bar graphs represent the means ± SEs. 10 neurospheres were observed for each experiment. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. (C)34: Compound 34. Scale bars: 100 μm. The neurospheres were derived from the iPSC line 201B7.