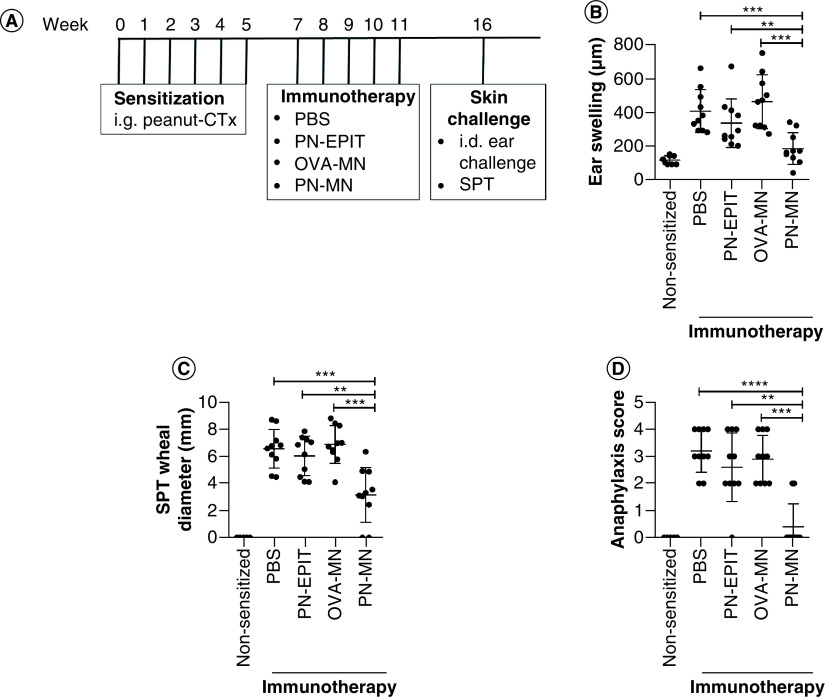
Figure 3. . Effects of peanut-coated microneedles on acute allergic skin response.
(A) Experimental schedule. Mice were sensitized orally with peanut and cholera toxin weekly for 6 weeks. 2 weeks later, sensitized mice received 5 weekly treatments with PBS (sensitized control), peanut (PN-EPIT), OVA-MNs or PN-MNs. (B) Mice were challenged intradermally into the ear with peanut, and ear swelling was measured 1 h after challenge. (C & D) Mice were challenged intradermally into the skin on the back and (C) the wheal diameter was measured 20 min after injection. (D) Symptoms of anaphylaxis were monitored for 60 min after challenge. n = 10 mice/group. Data are presented as mean ± standard error of the mean.
*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
MN: Microneedle; OVA: Ovalbumin; OVA-MNs: OVA-coated MNs; PBS: Phosphate-buffered saline; PN-EPIT: Epicutaneous peanut protein immunotherapy; PN-MNs: Peanut-coated MNs.