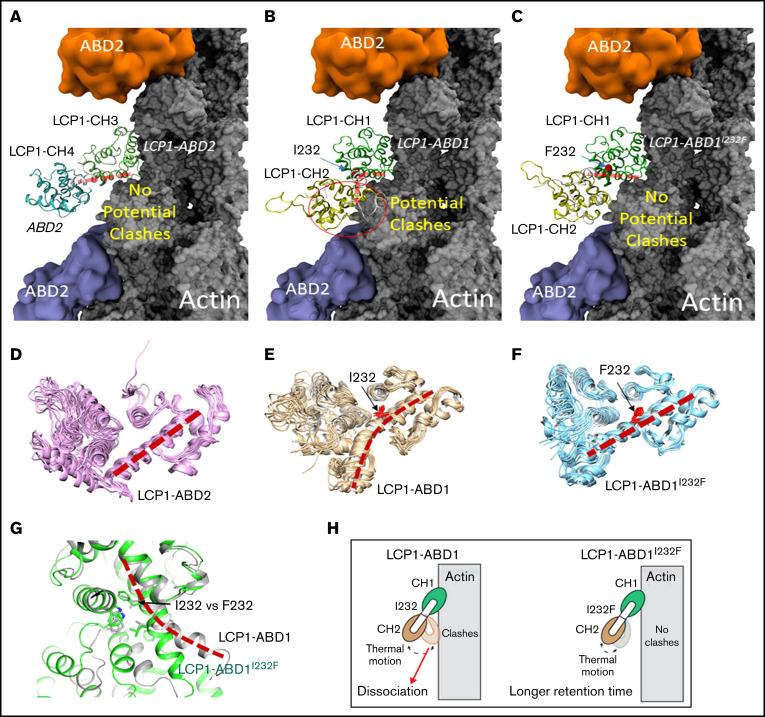
Figure 2.
Structural modeling of the LCP1 I232F mutation. (A) Cryo-EM structure of LCP1-ABD2 (Protein Data Bank identifier, 6VEC). Final snapshots of the molecular dynamics–generated LCP1-ABD1 (B) and LCP1-ABD1I232F (C) structure aligned to LCP1-ABD2 to model the binding between LCP1-ABD1 and F-actin. Ten representative conformations of LCP1-ABD2 (D), LCP1-ABD1 (E), and LCP1-ABD1I232F (F) obtained from 40 ns of molecular dynamics simulations are shown to depict the dynamic motion of the protein. The interdomain helices between CH1 and CH2 in LCP1-ABD1 and between CH3 and CH4 in LCP1-ABD2 are highlighted by red dashed lines. I232 and F232 are shown in red stick models. Potential clashes between LCP1-ABD1 and F-actin is indicated in a red circle. (G) Alignment of LCP1-ABD1-CH1 and LCP1-ABD1I232F-CH1. The bent interdomain helix in LCP1-ABD1 is depicted in a red dashed line. (H) A model illustrates the effect of I232F mutation to LCP1-ABD1 on the binding of LCP1-ABD1 to F-actin.