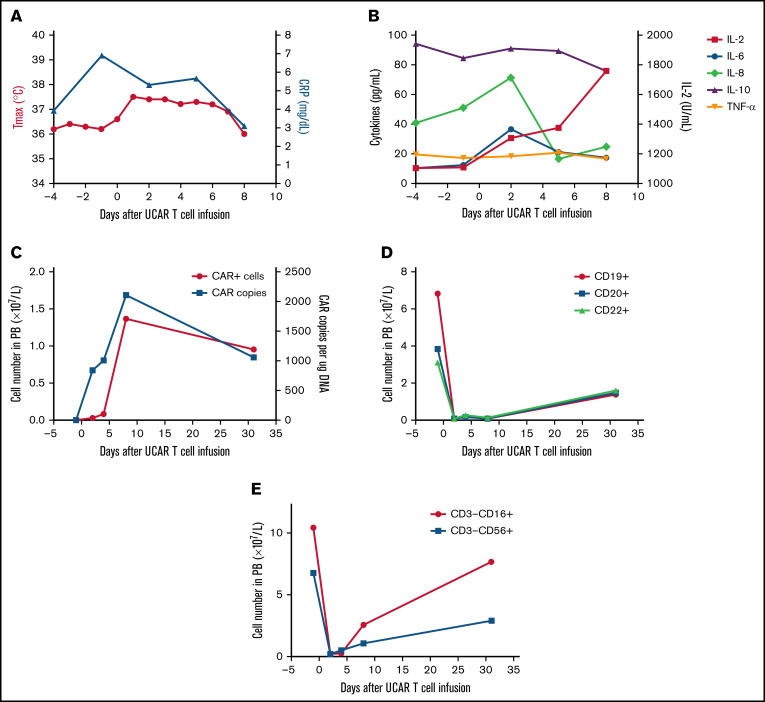
Figure 2.
Toxicities, persistence, and response in patient 2 after administration of universal CAR T cells. (A) The change in maximum temperature (Tmax) and the serum level of C-reactive protein (CRP) were monitored in patient 2 before and after universal CAR (UCAR) T-cell infusion, and Tmax and CRP recovered without any treatment. (B) The serum levels of cytokines, including interleukin-2 (IL-2), IL-6, IL-8, IL-10, and tumor necrosis factor-α (TNF-α), were tested before and after UCAR T-cell infusion. (C) Persistence of the infused UCAR T cells in peripheral blood (PB) of patient 2 before and after cell infusion. Flow cytometry and quantitative polymerase chain reaction were used to detect the level of UCAR T cells in PB. (D) The change in B-cell number before and after UCAR T-cell infusion. (E) The change in NK cell number before and after UCAR T-cell infusion.