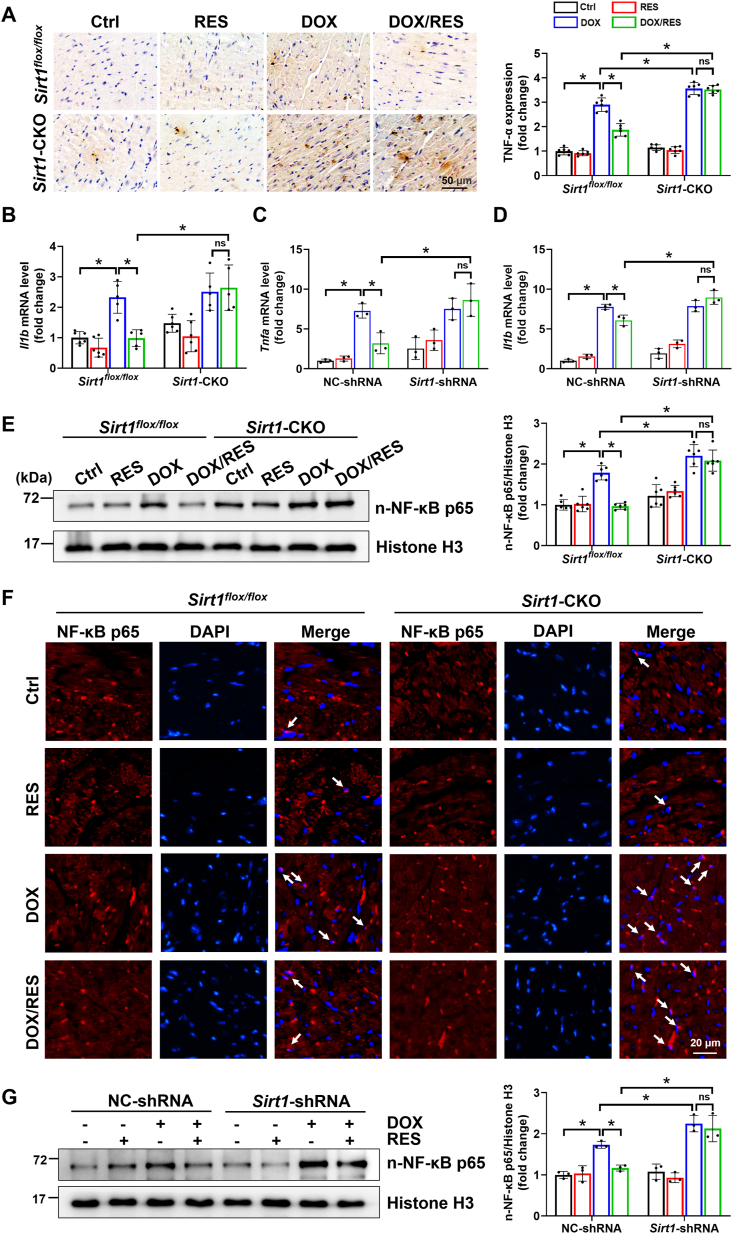
Fig. 2.
The activation of SIRT1 by RES improved DOX-induced inflammation in mice hearts and H9c2 cells. (A) Cardiac inflammatory response was detected by IHC staining for tumor necrosis factor-α (TNF-α, brown considered positive staining) followed by a quantitative analysis of the positive stains (n = 6). (B) Relative myocardial interleukin-1β (Il1b) mRNA level was measured by RT-qPCR (n = 5–6). (C, D) H9c2 cells were transfected with NC-shRNA or Sirt1-shRNA for 24 h, and then treated with RES (20 μM) or DOX (1 μM) or both for 24 h. Relative Tnfa and Il1b mRNA levels were measured by RT-qPCR. Three independent experiments were performed. (E) Western blot analysis and densitometric quantification of nuclear nuclear factor κB p65 (NF-κB p65) expression in cardiac tissues of each group (n = 6). Histone H3 as an internal control. (F) Nuclear accumulation of NF-κB p65 (indicated by white arrows) determined by immunofluorescent staining with anti-NF-κB p65 antibody (red) in cardiac tissues. (G) Western blot analysis and densitometric quantification of nuclear NF-κB p65 expression in H9c2 cells of different groups. Three independent experiments were performed. Histone H3 as an internal control. Data were presented as mean ± SD. *P < 0.05, ns indicates no significance. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)