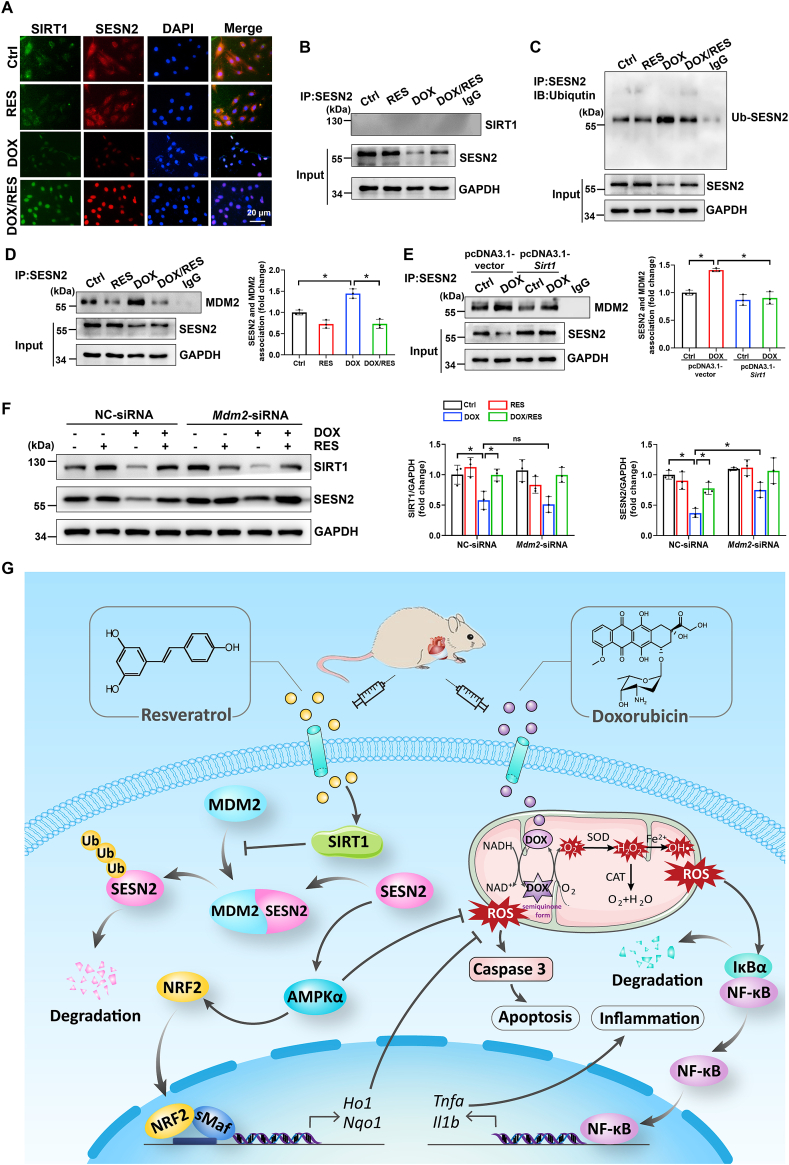
Fig. 7.
SIRT1 activation could reduce DOX-induced SESN2 ubiquitination possibly by disturbing the interaction of SESN2 and mouse double minute 2 (MDM2) in cardiomyocytes. (A) Co-localization of SIRT1 and SESN2 in H9c2 cells was determined by immunofluorescent staining. (B) Protein-protein interaction between SIRT1 and SESN2 in primary cardiomyocytes demonstrated by co-IP. (C) The immunoblotting of immunoprecipitated SESN2 with antibody recognized ubiquitin in H9c2 cells. (D, E) Protein-protein interaction between SESN2 and MDM2 in primary cardiomyocytes was demonstrated by co-IP with densitometric quantification. Three independent experiments were performed. (F) Western blot analysis and densitometric quantification of SIRT1 and SESN2 after transfection with NC-siRNA or Mdm2-siRNA in primary cardiomyocytes of different groups. Three independent experiments were performed. (G) Schematic illustration of a novel protective mechanism by SIRT1 activation to improve DOX-induced cardiotoxicity through SESN2/AMPKα pathway. GAPDH as an internal control. Data were presented as mean ± SD. *P < 0.05, ns indicates no significance.