FIG 3.
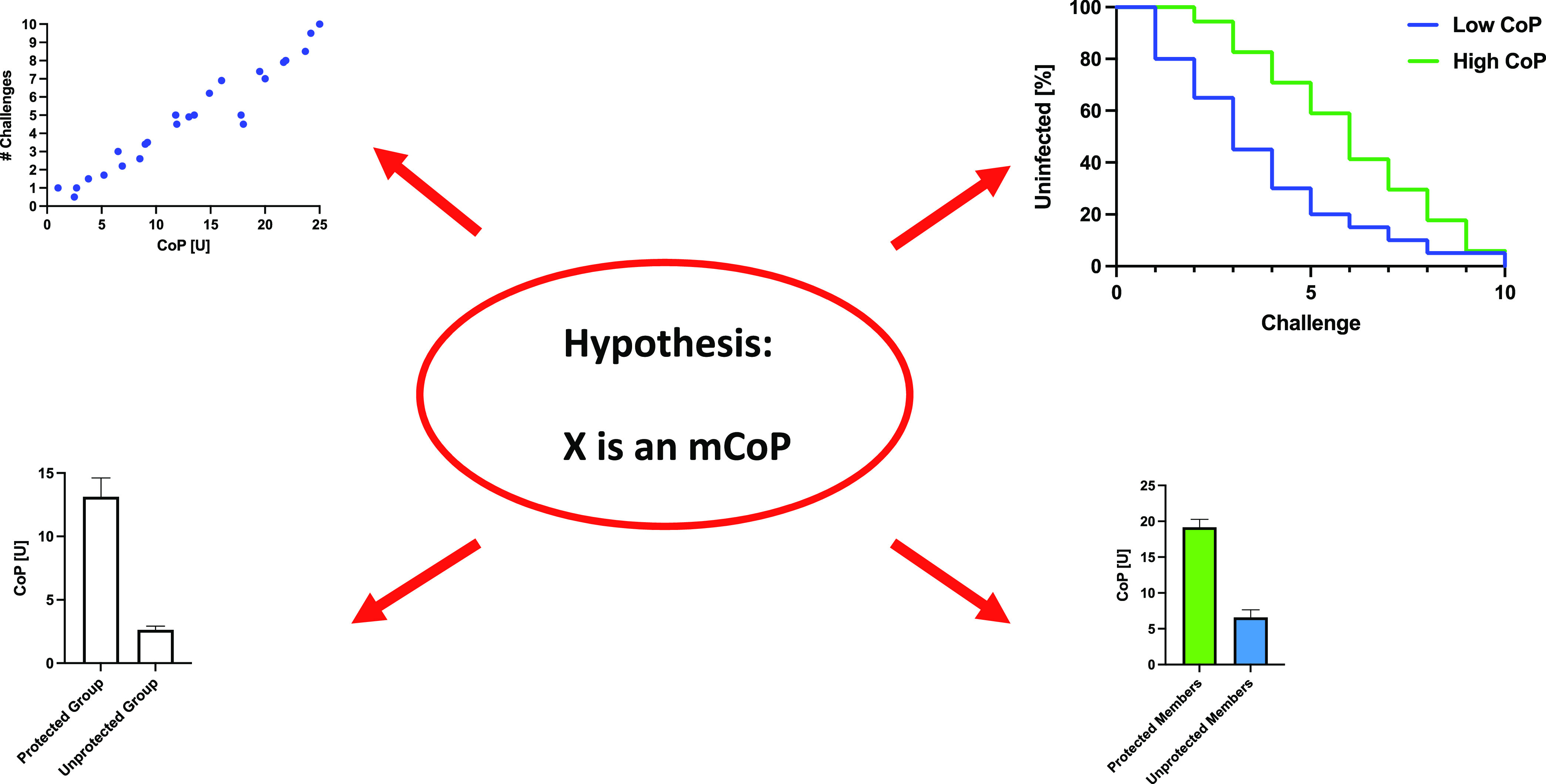
Test implications of hypothetical mCoPs. The application of systems biology in the search for CoPs and CoRs has been considered non-hypothesis-driven (98, 99). Once a factor is proposed to be an mCoP, it constitutes a hypothesis that can be tested. The arrows around the hypothesis represent implications that must hold up or the simple hypothesis is refuted. The quantified CoP (given on the x axis in imaginary units, U, in the upper left diagram) should correlate well with the number of virus challenges required for infection of study animals (upper right). To be relevant to protection, a CoP should be measured in samples taken close in time to the period during which protection is analyzed: causality implies stronger correlation for time-matched samples than for those from earlier or later time points. Stratification of these animals according to high CoP and low CoP values should give distinct Kaplan-Meier curves, showing more rapid infection in the low-CoP group (upper right). When there is more than one vaccine group and one shows net-protection while another does not, the measured CoP values should be higher in the protected group (lower left). Among the animals in a net-protected group or subgroup, individuals that become infected should have lower CoP values than those that stay uninfected (lower right). Even if the CoP candidate passes all those tests, however, it could still be an nCoP. Further in vitro experimentation is required to corroborate that the CoP is a mechanistic factor directly conferring protection. As an example, systems biological analysis of human responses to seasonal influenza vaccines showed that TLR5 expression was associated with the strength of virus-specific antibody responses. Experimentally, flagellin in murine gut microbiota was then shown to act as an adjuvant by signaling through TLR5. A similar mechanism was ultimately established in humans in that perturbing the microbiome affected the responses to influenza virus in a vaccine trial (97, 98, 169, 170).
