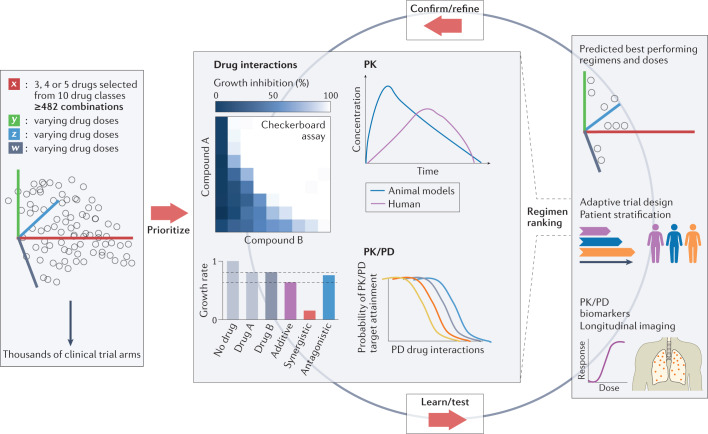
Fig. 4. Regimen prioritization.
Schematic illustration of the large number of possible combinations if drug candidates selected from 10 drug classes shown in Fig. 3 are combined in 3, 4 or 5 drug regimens (a minimum of 482 combinations assumes only 1 drug per class). In addition, owing to the varying drug doses, varying treatment durations and varying dosing frequencies, the number of clinical trial arms is within the thousands. Resource considerations underline the need for prioritization, using validated in vitro assays, drug interaction platforms, such as INDIGO or DiaMOND, pharmacokinetic (PK) and pharmacodynamic (PD) studies in preclinical species, and translational modelling tools, to select drug combinations with the highest potential to reduce treatment duration and improve cure in patients with tuberculosis, thus reducing the number of clinical trial arms to practical dimensions. New strategies, such as adaptive trial designs, doses and treatment duration tailored to patient characteristics, and longitudinal biomarkers of efficacy are required to accelerate the learning cycle, validate the in vitro and in vivo prioritization tools, and refine the computational approaches. Middle panel, top right, adapted from ref.188, Springer Nature Limited. Middle panel, bottom left, adapted from ref.189, Springer Nature Limited. Middle panel, top left, reprinted from ref.190, Springer Nature Limited.