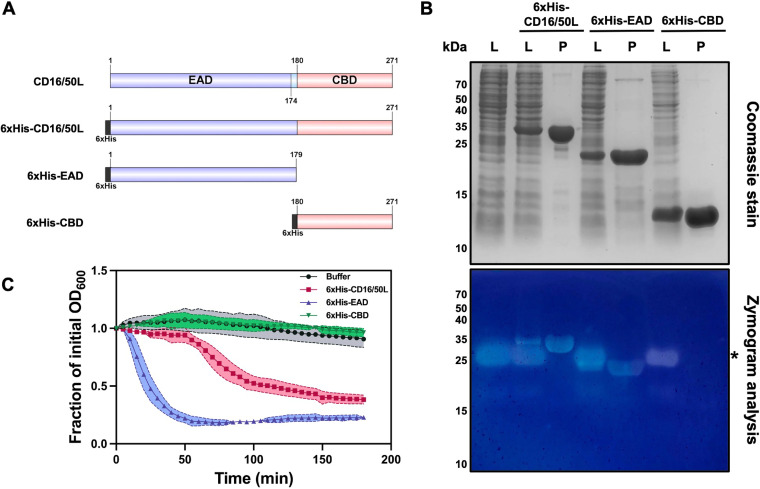
FIG 1.
The endolysin CD16/50L is a modular cell-wall hydrolase. (A) Schematic representation of domain structure of endolysin CD16/50L and N-terminally hexahistidine (6xHis)-tagged CD16/50L variants used in this study. CD16/50L is composed of the N-terminal enzymatically active domain (EAD) and the C-terminal cell-wall binding domain (CBD). Numbers indicate amino acid positions in the full-length protein. (B) The EAD of CD16/50L confers a peptidoglycan hydrolase activity. Recombinant protein variants of CD16/50L were expressed in E. coli. Crude lysate (L) and purified proteins (P) were resolved by SDS-PAGE followed by Coomassie blue staining or zymogram analysis using C. difficile peptidoglycan as substrate. Asterisk indicates a false positive band of a highly positively charged protein (see also Fig. S1C; [42]). (C) The full-length and EAD of CD16/50L are able to lyse intact cells of C. difficile. Cytolytic activity of endolysin was assessed by using turbidity reduction assay. Exponentially growing cells were harvested and resuspended in 50 mM Tris-HCl (pH 7.4) in an absence or presence of 2.5 μM purified CD16/50L variants and incubated at 37°C in anaerobic condition for 180 min. The decrement of OD600 over time was monitored and plotted. Mean ± SD are shown (n = 3).