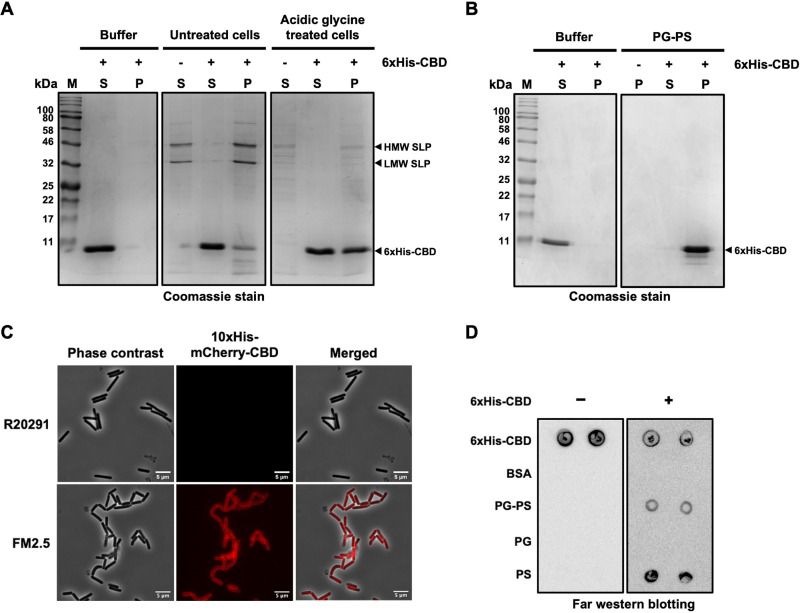
FIG 2.
The CBD of CD16/50L interacts with surface polysaccharide of C. difficile. (A) The CBD of CD16/50L binds to cells lacking the surface layer (S-layer) more effectively than the intact cells. Binding of the CBD to whole bacterial cells was investigated using a cell-based pulldown assay. Purified 6xHis-CBD was mixed with buffer, untreated C. difficile, or cells treated with acidic glycine buffer. After incubation and centrifugation, fractions of cell pellet (P) and supernatant (S) were separated in SDS-PAGE, followed by Coomassie blue stain. The high molecular weight (HMW), low molecular weight (LMW) subunits of surface-layer protein (SLP) and the purified 6xHis-CBD are indicated by arrowheads. (B) The CBD of CD16/50L binds to purified peptidoglycan and polysaccharide (PG-PS) complex. Analysis similar to Fig. 2A, but purified PG-PS complex was used. (C) The CD16/50L CBD localizes to the periphery of C. difficile cells deficient in functional S-layer. Affinity purified mCherry-fusion CBD was incubated with exponentially growing wild-type (R20291) or S-layer-deficient (FM2.5) C. difficile cells at 37°C for 30 min. Fluorescence microscopy shows the localization of CBD on the periphery of FM2.5 cells but not that of the R20291. The scale bar represents 5 μm. (D) The CBD of CD16/50L interacts with the secondary polysaccharide of C. difficile cell wall. Purified PG-PS complex, PG, and PS were spotted onto nitrocellulose membrane. Far Western blotting was performed by incubated the membrane with (right panel) or without (left panel) purified 6xHis-CBD in TBS-T containing 2% bovine serum albumin (BSA). Bound CBD was detected by anti-His antibodies followed by HRP-conjugated secondary antibodies and chemiluminescence detection. Spotted 6xHis-CBD and BSA serve as positive and negative controls of far Western blotting assay.