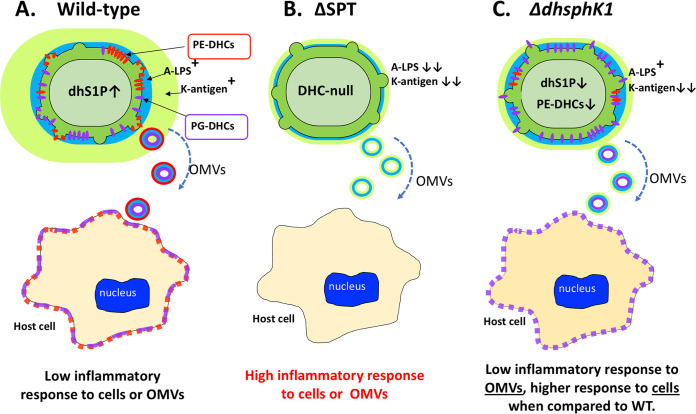
FIG 9.
Working model showing the link between synthesis of dhS1P and sphingolipids (PE-DHCs and PG-DHCs) and the ability of P. gingivalis to elicit an inflammatory response in THP-1 cells. (A) Our studies have shown that sphingolipids produced by the wild-type strain W83 are delivered to host cells (THP-1) via outer membrane vesicles (OMVs) or cell-to-cell contact and that this correlates with a low inflammatory response to W83. (B) In contrast, the W83 ΔSPT mutant, which is unable to synthesize any of the sphingolipids or dihydrophingosine-1-phosphate (dhS1P), elicits a robust inflammatory response to both the OMVs and cells. (C) Higher levels of PG-DHCs in relation to PE-DHCs produced by the ΔdhsphK1 mutant correlate with a high level of tolerance (elicits a low inflammatory response) to its OMVs, and yet, surprisingly there is a slightly higher response to ΔdhsphK1 cells than to the parent strain. We posit that dhS1P (which is anti-inflammatory) is contained within the cytoplasm of P. gingivalis and that this signaling molecule is released upon lysis or is transported out of the cell, and hence, there is a stronger inflammatory response to the ΔdhsphK1 mutant cells than to the parent strain. 75- by 2.1-mm ACE Excel 2 C18 column heated.