Table 9.
Structures and activity of the vitamin C derivatives against hyaluronidase.65
| Compound | Substituent |
(µM) or % inhibitiona, pH 5.0 |
||||
|---|---|---|---|---|---|---|
| SagHyal4755 | BTH | |||||
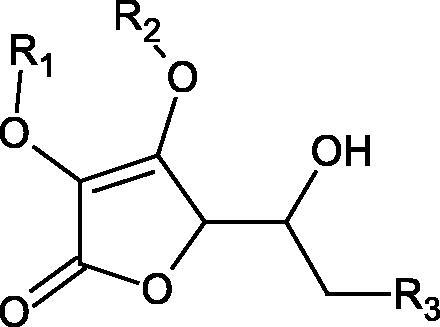
|
1 | H | H | H | 6100 | Inactive |
| 6 | Me | Me | H | Inactive | Inactive | |
| 7 | Bn | Bn | H | 355 | Inactive | |
| 8 | H | 24% (2000) | Inactive | |||
| 9 | Me | Me | 5% (160) | Inactive | ||
| 10 | Bn | Bn | Inactive | Inactive | ||
| 11 | 32% (190) | Inactive | ||||
| 13a | H | H | 43% (1100) | Inactive | ||
| 13b | H | H | 475 | Inactive | ||
| 13c | H | H | 772 | Inactive | ||
| 13d | H | H | 102 | 1380 | ||
| 13e | H | H | 72 | 580 | ||
| 13f | H | H | 47 | 208 | ||
| 13g | H | H | 14.3 | 96 | ||
| 13h | H | H | 8.4 | 71 | ||
| 13i | H | H | 4.2 | 57 | ||
| 13j | H | H | 0.9 | 39 | ||
| 13k | H | H | 132 | 33% (1430) | ||
| 13l | H | H | 358 | 2006 | ||
| 13m | H | H | 717 | Inactive | ||
| 13n | H | H | 437 | Inactive | ||
| 13o | H | H | 61 | 188 | ||
| 13p | H | H | 102 | 543 | ||
| 13q | H | H | 280 | Inactive | ||
| 13r | H | H | 76 | 210 | ||
| 13s | H | H | 31 | 105 | ||
| 13t | H | H | 7.5 | 37 | ||
Bn: benzyl group.
