Abstract
Background
When it comes to critical early post-acute TIA/stroke phase, there is a lack of a comprehensive multi-parametric telemonitoring system. The COVID-19 emergency, its related global mobility restrictions and fear of hospitalization further highlighted the need of a comprehensive solution.
Objective
We aimed to design and test a pragmatic e-Health system based on multiparametric telemonitoring to support of TIA/stroke patients in sub-acute phase during the COVID-19 pandemic.
Methods
We proposed a telemonitoring system and protocol for TIA/minor stroke patients during COVID-19 pandemic for patients at risk of stroke recurrence. This system involves the use of portable devices for BP/HR/SpO2/temperature sensing, panic-button, gateway, and a dedicated ICT platform. The protocol is a 14-day multiparametric telemonitoring, therapy, and emergency intervention based on vital sign alteration notifications. We conducted a proof-of-concept validation test on 8 TIA/minor stroke patients in the early post-acute phase (< 14 days from ischemic event).
Results
The proposed solution allowed to promptly and remotely identify vital sign alterations at home during the early post-acute phase, allowing therapy and behavioral intervention adjustments. Also, we observed a significant improvement of quality of life, as well as a significant reduction of anxiety and depression status. TUQ showed ease of use, good interface quality and high user satisfaction of the proposed solution. The 3-month follow-up showed total adherence of prescribed therapy and no stroke/TIA recurrence or other emergency department admissions.
Conclusion
The proposed e-Health solution and telemonitoring protocol may be highly useful for early post-acute remote patient management, thus supporting constant monitoring and patient adherence to the treatment pathway, especially during the COVID-19 emergency.
Keywords: e-Health, Telemonitoring, TIA, Stroke, COVID-19
1. Introduction
As a result of the ongoing COVID-19 pandemic most of the world population is currently subject to mobility restrictions [1] and further restrictive measures imposed by national health authorities. In this emergency, the attention of healthcare providers and health authorities is primarily focused on infected patients and frontline responders. The emergency has led health care systems to face a major burden, with a rise in health care services requests beyond the current hospitals' capacities: from the number of hospitalized patients, to a lack of intensive care beds and other resources, not to mention the need for more workforce. However, invalidating pathologies should not be neglected and fall out of focus.
Stroke is the second most common cause of death in the world after ischemic heart disease, as well as the most common cause of adult disability in developed countries [2]. During the first months of the pandemic emergency we observed a consistent reduction of admissions in our Hub Stroke Unit compared to the same period in 2019 [3,4], especially due to the lower number of transient ischemic attack (TIA) and minor strokes admission, which may have been related to the widespread fear of going to the hospital during the pandemia when showing mild or transient stroke symptoms. Similar findings were observed in other stroke units worldwide [[5], [6], [7]]. The difficulties in performing all the diagnostic etiology exams (that may involve other specialists) and the risk of possible intra-hospital SARS-CoV-2 infection of stroke/TIA patients often leads to a shorter hospitalization and patients are more frequently discharged without a complete stroke work-up [3].
TIA and Stroke patients are at high risk of stroke recurrence, with this risk being greater during the first 3 months following an ischemic event [8]. In particular, the cumulative risk in stroke patients is increasing from 3% during the first month to 40 % at 10 years after the event [9]. TIA patients are also at high risk for stroke recurrence, with a cumulative stroke risk of 3% during the first month [10], and 18 % at 10 years [11].
Alteration of vital signs such as blood pressure, heart rate, body temperature, saturation and respiration rate may be correlated with cerebrovascular risk factors and may thus be useful to assess patient condition, titrate therapy, and prevent adverse outcomes. Among which raised blood pressure (BP) is the most important reversible stroke risk factor. Telemonitoring has been proved to be effective to achieve BP reduction in a general hypertensive population [12,13]. Yet, its sensitivity to context complicates the implementations [14]. The benefits observed in a single risk factor telemonitoring intervention in the general population could be more challenging if applied to patients with sub-acute minor Stroke/TIA as they may be more debilitated [15] with more complex polypharmacy than the other population. To improve management of sub-acute and chronic patients, as well as the psychological aspects, comprehensive, pathology- and patient-specific solutions are necessary [16].
Telemonitoring and teleassistance solutions carried out through smart wearable technologies may assure patients' safety in the post-acute phase outside the hospital, which became even more important against the background of the COVID-19 emergency [17] in the way patients access the healthcare system [3,18]. Vital sign sensors have been used extensively to monitor physiological effects, differently from the most part of the studies and interventions in which single-parameter devices are used [19]. However, only a few studies focused on telemonitoring of post-stroke patients. Home-based monitoring and rehabilitation were studied in the sub-acute and chronic stroke phase, proving to be feasible and effective [[20], [21], [22], [23]]. Nevertheless, these studies do not focus on the early post-stroke phase (< 14 days from ischemic event). In addition, the COVID-19 emergency is unprecedented in terms of global mobility restrictions and fear of hospital admission, boosting even more the need for a comprehensive solution.
We aimed to design, implement and test a pragmatic e-health system for multiparametric telemonitoring and to support minor stroke/TIA patients the early post-acute phase (< 14 days from ischemic event) during the emergency and reduced mobility related to the COVID-19 pandemic.
2. Methods
2.1. Proposed remote patient monitoring e-Health solution and protocol
The proposed telemonitoring and teleassistance solution and protocol for TIA/minor stroke patients were developed during COVID-19 pandemics for adult patients recently (< 14 days) affected by TIA at moderate risk of stroke recurrence (ABCD2 4–5) or by minor ischemic stroke (NIHSS ≤ 5). The following subsections describe the patients’ standard acute TIA/minor stroke management protocol as well as the proposed e-Health post-acute solution and relative telemonitoring protocol.
2.1.1. Acute management of TIA/minor stroke patients
Neurological assessment, non-enhanced CT, ECG, as well as administration of antiplatelet agent/anticoagulant therapy (if applicable), were immediately performed when patients presenting at the ED showed acute focal and transient neurological symptoms potentially compatible with TIA/minor stroke. Extracranial CT angiography was performed in patients presenting with recurrent focal neurological symptoms or cardiovascular risk factors. After ABCD2 risk stratification, in case of high score (score 6–7), patients were hospitalized in the stroke unit; in case of low or moderate score (score ≤5), patients were rapidly (<48 h) referred to our TIA service. At TIA service admission (or during stroke unit hospitalization), a series of checks were performed: neurological visit, especially focusing on vascular risk factors, based on patients’ age, hematologic laboratory tests, ECG, EEG, extracranial and intracranial arteries color-Doppler. When necessary, echocardiography, 48 h Holter ECG and MRI were performed at a later point. Once the clinical picture and the inclusion/exclusion criteria had been assessed, at discharge from the Stroke Unit/TIA service patients were proposed to enroll in a 2 week telemonitoring program starting within 14 days from the ischemic event.
2.1.2. e-Health solution and post-acute telemonitoring protocol
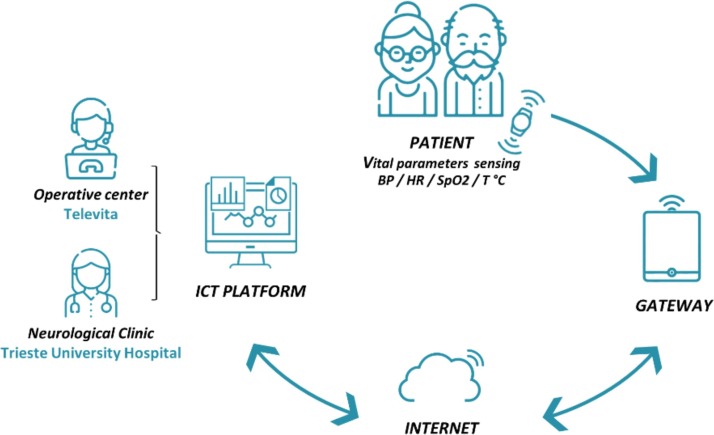
Fig. 1 illustrates the proposed telemonitoring system. This system consists of portable devices for BP, HR, SpO2 and temperature vital signs sensing, panic button, getaway and a dedicated ICT platform. The devices were configured to gather vital parameter data and to dialogue with the gateway. Blood pressure was recorded by using blood pressure monitoring device (Diamond Cuff BP - P80, ForaCare), SpO2 were acquired by pulse oximeter (OxyWatch, ChoiceMMed), heart rate (HR) by using both aforementioned devices and body temperature was sensed by thermometer (FORA IR21, ForaCare). The Bluetooth wireless connection was the protocol used between sensing devices and gateway. The gateway handles communication with the server application exploiting mobile broadband connectivity. The acquired data were sent throughout the gateway to the healthcare provider's ICT platform and supplied the necessary input to the various front-ends to visualize the raw and processed data and provide smart notifications to the Televita Operative center and dedicated service of Trieste University Hospital Neurological Clinic.
Fig. 1.
Schematic diagram of the proposed telemonitoring system.
The activation of the service for each user was made by adding data and parameters for the patient configuration in the structured form within the platform and containing: anagraphic data (date of birth, social security number, residence, etc.), anthropometric data (height, weight, BMI), clinical data of current and past pathologies and therapy prescribed. In addition, data and contact of formal/informal caregivers and healthcare network (who to contact in case of non-serious need or in case of no response from the user), general practitioner, reference stroke specialist physician were inserted in the platform.
The e-Health remote patient monitoring system including devices (vital signs sensors), gateway (tablet) and panic button was delivered at the patient's home and the system was set up by a technician protected by personal protection equipment (PPE). During this single access at their home, patients (if self-sufficient) and/or caregiver received 1 -h training, too. Physicians were remotely trained to use the platform.
For each patient, the frequency and schedule of the remotely measured vital signs (blood pressure, heart rate, SpO2 and temperature) were defined. For each parameter personalized patient-specific thresholds for minor and serious alarms were defined and set to be automatically monitored by the system. Customized patient-specific procedures for exceeding the thresholds (minor and serious thresholds) were defined. In particular, it was possible to set a personalized intervention procedure in case of mild exceeding (minor alarm) for two or three consecutive times of a specific parameter or the concomitant exceeding of two different parameters (e.g, BP and HR, Temperature and SpO2). Each measurement was registered in the portal and, together with highlighted exceedings, was available for operator (in service center) and physician (in the TIA service) review and intervention. The system was set to automatically notify the operator the exceedings defined by personalized intervention procedure (consecutive, concomitant or severe exceedings). The operator would in turn fast check their reliability and assign them to the stroke specialist for the management, including behavioral advice or therapy changes. In particular, for serious threshold exceedings (possible life-treating conditions or severe complications) the operator immediately verifies the state of health and reliability of the measurement with a phone call to the patient/caregiver the referring specialist physician is immediately notified via an SMS/telephone call. Based on physician feedback, there was the possibility to call for an emergency and to send an ambulance to the patient's home. In addition, patients were provided with a panic button to alert an emergency and with a device (tablet) with video teleconferencing features to enable them to have medical face-to-face communication. The videoconferencing feature may not work in rural locations, especially mountain areas where mobile broadband may be limited.
The system was managed and supported by the service center and trained operators with the aim of offering psychosocial support, monitoring of personal and environmental situations, promoting correct and healthy lifestyle habits. The service center ensured that users use the supplied data devices correctly and make regular scheduled parameter measurements. The service center also verified possible problems concerning the failure of the user to measure by contacting him by phone 30 min after the scheduled time (e.g. the user has forgotten to carry out the measurement; failure/equipment or other possible problems) and by providing the necessary support for the correct use of the service (technical and at the same time psychological support) favoring constant monitoring and patient adherence to the treatment path.
At the end of the sub-acute telemonitoring period stroke, specialists released a report and contacted the patients in order to inform them of telemonitoring summary outcome and to prescribe the adequate therapy and follow-up visit at the neurovascular specialist clinic. Concomitantly, the devices were withdrawn by the technician and all devices were adequately sanitized.
2.2. Evaluation of proposed telemonitoring solution
2.2.1. Study population and evaluation study protocol
We included patients with a recent (< 14 days) diagnosis of transitory ischemic attack (TIA) or minor ischemic stroke (NIHSS ≤ 5) admitted to our TIA service / Stroke Unit University Medical Hospital of Trieste (Italy) between 9 March 2020 (start of Italy lockdown) and 9 April 2020 (COVID-19 lockdown period). Other inclusion criteria were: patients over 18 years of age, ABCD2 score = 4–5, at least one of major cerebrovascular risk factors (arterial hypertension, diabetes, atrial fibrillation, dyslipidemia, active smoke). The patients who refused the service and/or or were unable to consent or use self-monitoring equipment by themselves or without easy access to help were excluded.
During TIA service/stroke admission, most of the patients underwent a common neurologic TIA/stroke work-up including assessment of stroke risk factors, admission and follow-up neuroimaging assessment, ECG, EEG, intracranial and extracranial ecocolordoppler, echocardiography and Holter-electrocardiography or telemetric ECG-monitoring. Patient with acute minor stroke who were eligible for thrombolysis were treated with intravenous rtPA accordingly standard guidelines [24].
Enrolled patients received the devices at home together with one hour training for using the devices and services described in the previous section 2.2. The patient telemonitoring and teleassistance lasted 14 days and were performed according to the proposed protocol described in detail in section 2.1.
To evaluate the proposed solution, the following data were gathered and analyzed together with questionnaires administration. Stroke related clinical features at enrollment were collected: (1) demographic details (e.g., age, sex); (2) TIA/Stroke etiology by TOAST classification [25]; (3) TIA/Stroke syndrome by Bamford classification (Total Anterior Circulation Infarct, TACI; Partial Anterior Circulation Infarct, PACI; Lacunar Stroke, LACI; Posterior Circulation Infarct, POCI); (4) National Institutes of Health Stroke Scale (NIHSS) score at acute hospital admission and at the beginning of telemonitoring/teleassistence; (5) TIA/stroke modified Rankin score (mRS) at the enrollment; (6) stroke risk factors (arterial hypertension, diabetes mellitus, dyslipidemia, ischemic cardiopathy, atrial fibrillation); (7) rTPA therapy; (8) time from TIA/stroke event to enrollment; (9) prescribed therapy at Stroke Unit/TIA service discharge.
Vital signs and parameters measured remotely during the telemonitoring, namely Blood Pressure, Heart Rate, SpO2 and temperature were collected from the ICT platform. The number of total events exceeding the threshold, including minor and serious alarms, was collected for each vital sign and patients. Furthermore, we evaluated the number of the patients' requests for remote technical and clinical assistance, as well as for telemonitoring compliance. During the telemonitoring/teleassistance period we registered the amount of: neurologist intervention and the modification of prescribed therapy; stroke recurrences; new emergency department admission for any other acute events during telemonitoring and during the following 3 months. Adherence to therapy and reaching the target of risk factors control were evaluated at 3 months. Telehealth Usability Questionnaire (TUQ) was submitted on the last day of telemonitoring to evaluate the usability and satisfaction of telehealth implementation and services [26]. At enrollment and at the end of the 14-day telemonitoring The Hospital Anxiety and Depression Scale (HADS) was administered to detect any anxiety and depressive status [27]. At the same time points, the 5-level EQ-5D version (EQ-5D-5L) was used to evaluate the health-related Quality of Life [28]. The scale measures quality of life on a 5-component scale including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.
This study was approved by the Local Ethics Committee (Comitato Etico Unico Regionale - CEUR, FVG, Italy) and conducted according to the principles of the Declaration of Helsinki. All participants released their informed consent.
2.2.2. Statistical analysis
All statistical analyses were performed through SPSS Statistics 23 (IBM, Armonk/NY, USA). We presented the continuous variables with a normal distribution as mean and standard deviations (SDs); variables with a skewed distribution were presented as median and interquartile ranges (IQRs) pointing out the 1 st and 3rd quartile, and categorical variables were presented as counts and percentages (%). The differences between pre and post scores were researched through parametric (paired t-test) and nonparametric (Wilcoxon signed-rank test) tests. Statistical significance was set at p < 0.05.
3. Results
The mean ± SD age of eight included patients (6 F/2 M) was 73.2 ± 9.6 (range 61–85). Five of them were patients who experienced minor ischemic stroke recently (2 of them treated with thrombolysis), three patients had experienced recent TIA event. Seven patients presented a PACI syndrome at hospital admission, while only one patient presented a POCI syndrome. All included patients presented a NIHSS between 0 and 5 at hospital admission, while at the time of telemonitoring enrollment, all patients had a NIHSS between 0 and 2, and mRS disability scale between 0 and 1. All patients presented at least one cardiovascular risk factor: arterial hypertension (n = 6; 75 %), atrial fibrillation (n = 4; 50 %), ischemic cardiomyopathy (n = 2; 25 %), diabetes (n = 3; 38 %), dyslipidemia (n = 5; 63 %), smoke (n = 3; 38 %). Considering TOAST TIA/Stroke etiology, 3 patients were cardioembolic, 4 cryptogenic, 1 atherothrombotic. Polytherapy was prescribed at hospital discharge in all patients (number of prescribed drugs range 4–11). Seven patients were taking beta-blocks therapy before the telemonitoring enrollment. The mean time from TIA/stroke event to enrollment was 9 ± 2.8 days (range 5–13). During telemonitoring period and up to the follow-up at 3 months, no stroke/TIA recurrence episodes were reported and patients did not present any other acute complication that required emergency department admission.
In Table 1 the relevant clinical features and the performance of telemonitoring/teleassistance are reported for each patient. Mean ± SD of the total number of exceeding the threshold events was 31.7 ± 10.8 (4.1 ± 4.0 and 27.6 ± 8.7, serious and minor alarms, respectively). 52 % of alarms were related to BP (31 % and 21 % BP over and under threshold, respectively), 34 % to HR (23 % and 11 % HR over and under threshold, respectively), 9% to lower SpO2 level and 5 % to higher body temperature. During the telemonitoring/teleassistance period the neurologist remote intervention was observed on average 2.2 ± 1.2 per patient. In 7 out of 8 patients the clinician changed the prescribed therapy: in three cases the neurologist increased antihypertensive therapy, three patients presented hypotensive episodes with consequent decrease of antihypertensive treatments, in one case the physician modulated the beta-blocks therapy for bradycardia and in one case double antiplatelet therapy was recommended following self-suspension. 6 out of 8 patients had a normalization of all parameters in the last two days of study period. The remaining two patients presented a progressive reduction in blood pressure in the last two days of telemonitoring/teleassistance period.
Table 1.
Baseline clinical characteristics and performance of telemonitoring/teleassistance for each patient.
| Baseline |
Telemonitoring |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | AIS/TIA | NIHSS at enroll. | Enroll. day [days] | Med. prescr. [n] | Severe alarms [n] | Mild alarms [n] | Alarms physic. [n] | Physic. to patient [n] | Missing measur. [n] | Tech. assist. [n] | Therapy modif. [n] |
| 1 | AIS | 0 | 9 | 4 | 7 | 33 | 8 | 2 | 0 | 6 | 1 |
| 2 | TIA | 0 | 6 | 8 | 0 | 22 | 10 | 1 | 0 | 2 | 1 |
| 3 | AIS | 0 | 11 | 7 | 12 | 26 | 12 | 3 | 0 | 4 | 1 |
| 4 | AIS | 1 | 13 | 8 | 1 | 20 | 6 | 4 | 2 | 1 | 2 |
| 5 | AIS | 1 | 10 | 8 | 2 | 34 | 11 | 3 | 1 | 3 | 1 |
| 6 | AIS | 2 | 11 | 4 | 1 | 15 | 4 | 1 | 4 | 3 | 0 |
| 7 | TIA | 0 | 5 | 11 | 5 | 42 | 18 | 1 | 1 | 0 | 1 |
| 8 | TIA | 0 | 7 | 6 | 5 | 29 | 11 | 3 | 0 | 0 | 1 |
| Mean ± SD | – | 0.5 ± 0.7 | 9 ± 2.7 | 7.0 ± 2.3 | 4.1 ± 4.0 | 27.6 ± 8.7 | 10.0 ± 4.2 | 2.2 ± 1.2 | 1.0 ± 1.4 | 2.4 ± 2.0 | 1.0 ± 0.5 |
Notes: AIS: acute ischemic stroke; TIA: transitory ischemic attack; NIHSS at enroll.: National Institutes of Health Stroke Scale at enrollment. Enroll. Day: Timespan from Cerebrovascular event to telemonitoring enrollment. Med. Prescr.: Number of Medications Prescribed. Severe Alarms. Mild Alarms. Alarms physic: Alarms Assigned to the physician by operator. Physic. to patient: Physician call to the patient. Missing measur.: Missing Vital signs Measurements. Tech. assist.: Technical assistance oparator intervention. Therapy modif.: Therapy modifications.
The patients were highly compliant with the measurement tasks, missing on average only one task out of overall 42 multiparametric measurement tasks. A low number of patient’s requests for remote technical assistance was observed (2.4 ± 2.0). Despite being available, no patient asked for a video call with a physician during the trial.
Follow-up at 3 months showed a total adherence of therapy prescribed during the telemonitoring period. In 7 out of 8 patients the cerebrovascular risk factor management targets were reached.
Table 2 reports the scores of the 5-level EQ-5D version (EQ-5D-5L) test and the Hospital Anxiety and Depression Scale (HADS) at the enrollment and at the end of the 14-day telemonitoring, as well as their comparison. A significant improvement of quality of life measured with EQ-5D-5L was observed after 14 day of telemonitoring (p = 0.031), as well as a significant reduction of anxiety (p = 0.016) and depression (p = 0.016) status.
Table 2.
Comparison of Scores of EuroQoL Quality of Life Scale (EQ-5D-5L) test and the Hospital Anxiety and Depression Scale (HADS) observed at enrollment (pre) and at the end of the 14-day telemonitoring period (post).
| Patient | EQ-5D-5L Score | EQ-5D-5L Score | EQ-5D-5L 0–100 scale | EQ-5D-5L 0–100 scale | HADS Anxiety | HADS Anxiety | HADS Depression | HADS Depression |
|---|---|---|---|---|---|---|---|---|
| (pre) | (post) | (pre) | (post) | (pre) | (post) | (pre) | (post) | |
| 1 | 0.80 | 0.85 | 70 | 90 | 6 | 2 | 2 | 0 |
| 2 | 0.85 | 1 | 90 | 90 | 6 | 3 | 2 | 0 |
| 3 | 0.59 | 0.69 | 50 | 60 | 2 | 1 | 2 | 2 |
| 4 | 0.85 | 0.85 | 70 | 75 | 1 | 0 | 1 | 0 |
| 5 | 0.62 | 0.69 | 50 | 60 | 11 | 11 | 13 | 9 |
| 6 | 0.32 | 0.71 | 55 | 60 | 8 | 7 | 9 | 7 |
| 7 | 0.66 | 0.85 | 50 | 70 | 3 | 2 | 6 | 3 |
| 8 | 0.85 | 0.85 | 40 | 50 | 12 | 6 | 7 | 6 |
| Median | 0.73 | 0.85 | 52.5 | 65 | 6 | 2.5 | 4 | 2.5 |
| (IQR) | (0.60–0.85) | (0.70–0.85) | (50–70) | (60–82.5) | (2.5–9.5) | (1.5–6.5) | (2–8) | (0.0–6.5) |
| pre vs post | p = 0.031 | p = 0.016 | p = 0.016 | p = 0.016 |
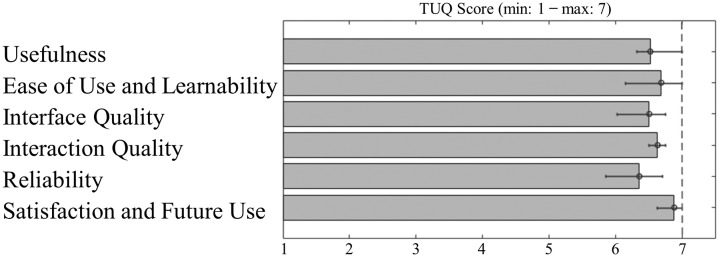
In Fig. 2 median and IQR values of results of Telehealth Usability Questionnaire are reported for each group of sub-items pertaining to the specific domain [26]. Patients’ graded the solution's usability as very high, easy to use, with a good interface quality and overall high satisfaction with it. Particularly, the patients reported a median score of “Usefulness” of 6.51 (6.31–7.00), a median score of “Ease of Use and Learnability” of 6.68 (6.15–7.00), a median score of “Interface Quality” of 6.50 (6.02–6.75), a median score of “Interaction Quality” of 6.63 (6.5–6.75), a median score of “Reliability” of 6.35 (5.85–6.7) and a median score of “Satisfaction and Future Use” of 6.87 (6.62–-7.00).
Fig. 2.
Results of Telehealth Usability Questionnaire (TUQ) to evaluate the usability and satisfaction of telehealth implementation and services. Median and IQR for each of questionnaire domains.
4. Discussion
Telemonitoring solutions supported by IoT and smart technologies may guarantee patients home care in the post-acute phase [17], which is crucial in restricted mobility scenarios such as the COVID-19 pandemic [29]. Despite advances in prevention and treatment, ischemic stroke is still one of the leading causes of death and the first cause of adult disability worldwide [2]. In particular, TIA and minor stroke patients are at high risk of stroke recurrence during the first weeks after the cerebrovascular event [10]. In the underlying feasibility study, we proposed and performed a preliminary evaluation of a pragmatic e-health system to carry out multiparametric telemonitoring and to support minor stroke/TIA patients in the early post-stroke phase (< 14 days from ischemic event). This is the first study focusing on telemonitoring of TIA/minor stroke patients in the early post-ischemic phase during the COVID-19 pandemic. In this emergency scenario, we remotely monitored vital signs of these patients, and in most cases the smart alerts allowed to modulate the therapy and to obtain a better control of cerebrovascular risk factors, in particular of hypertension.
The proof-of-concept evaluation test showed the proposed technological solution and telemonitoring protocol could be highly useful for early post-acute remote patient management. It provided the possibility to efficiently manage and remotely monitor minor stroke/TIA patients just after early discharge, allowing to reduce hospitalization stay without compromising patients' safety. The results were particularly significant considering the inclusion of TIA/minor stroke patients with advanced average age, multiple cerebrovascular risk factors, ongoing polytherapy and recent cerebrovascular event, who are at particular risk with regard to disability, quality of life, and anxiety.
The proposed solution also provided the possibility to better tailor post-acute therapy, allowing therapy modification in 7 of 8 patients based on the feedback provided by vital signs monitoring and concomitant exceeding threshold notifications. During the 14-day telemonitoring, 31.7 ± 10.8 events exceeding the threshold were observed per patient. In particular, 4.1 ± 4.0 and 27.6 ± 8.7 for serious and minor alarms, respectively. The most prevalent vital sign alarm was the alteration of BP (52 %), followed by (HR 9 %). Indeed, BP alterations are reported to be the most important reversible risk factor for recurrent stroke, with risk increasing by about one-third for every 10 mmHg increase in systolic BP [30]. Treatment of elevated blood pressure post cerebrovascular event reduces the risk of subsequent strokes, stroke deaths, and cardiovascular deaths [31]. In our 8-patient sample, the neurologist increased anti-hypertensive therapy for three patients, while three other patients presented hypotensive episodes with consequent decrease of anti-hypertensive treatments with a rapid normalization of blood pressure in the following days of telemonitoring period.
Single vital parameter monitoring has been used extensively in research to evaluate patients' physiological state. However, single-parameter monitors lacks of a broader, holistic health overview. Multi-parameter approach may allow better management of the risk factors and complications of a complex condition such as the TIA/minor stroke patients [17]. Our study was also based on monitoring other vital sign parameters beside BP, enabling therapy adjustments for the heart rhythm, as well as the antiplatelet therapy management.
In addition, we observed a significant improvement of quality of life after the two weeks of proposed telemonitoring service, as well as a significant reduction of anxiety and depression status, measured with EQ-5D-5L. This result is probably due to the fact that the patients felt cared and closely monitored, even at a distance, after an acute life-threatening event. This was furtherly reinforced by the possibility of discussing symptoms, therapy assumption, behavior measures and risk factors related to vital sign alterations. For this reason, despite formal video call visit was available for patients, none of them requested it.
The possibility of being followed at home and not having to go to the hospital several times in a peculiar time such as the COVID-19 pandemic was reassuring. The inclusion of SpO2 and body temperature monitoring furtherly reassured the patient. The current health emergency scenario linked to the COVID-19 pandemic requires the adoption of tangible and incisive actions to counter the spread of the infectious agent, such as avoiding as much as possible the movement of citizens from their homes, thus contributing to reduce overcrowding at health facilities.
The results of the TUQ assessment showed that patients graded the usability of the proposed technology as very high, reporting its ease of use, good interface quality and overall high satisfactions with the proposed solution. Indeed, the results also showed that, despite advanced age and recent acute event with possible involvement of higher cortical functions, the TIA/minor stroke patients were highly compliant with the measurements tasks and only a low number of patient’s requested technical assistance.
This preliminary study presents some limitations. This is a single center study on a limited study sample without control group. Despite the consecutive enrollment of the patients, the limited study sample may have increased the chance of selection bias. A larger study with a control group is needed to confirm these preliminary results on the efficacy of the proposed e-health system in the management of main cerebrovascular risk factors and secondary prevention, as well as to assess its effectiveness in preventing recurrent stroke.
5. Conclusion
In conclusion, in this study we proposed a pragmatic e-health system and protocol for multiparametric telemonitoring and support of minor stroke/TIA patients in the early post-acute phase (< 14 days from ischemic event). The proof-of-concept evaluation test showed that the proposed technological solution and telemonitoring protocol could be highly useful for early post-acute remote patient management to favor constant monitoring and patient adherence to the treatment path. The COVID-19 emergency is unprecedented as for global mobility restrictions and fear of hospitalization, thus urgently requiring a comprehensive solution to guarantee health care for patients.
Authors’ contributions
Author contributions included conception and study design (MA and GF), proposed system and protocol design (MA, GF, MN, AM and AA) data collection or acquisition (MA, GF, MN, PC, PP, AM), statistical analysis (MA and AA), interpretation of results (MA, GF, AA and PM), drafting the manuscript work or revising it critically for important intellectual content (MA, GF, AA and PM) and approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (All authors).
Sources of funding
This study did not receive any funding.
Summary points.
What was already known
-
•
Telemonitoring solutions may assure patients' safety in the post-acute phase outside the hospital.
-
•
When it comes to critical early post-acute TIA/stroke phase, there is a lack of a comprehensive multi-parametric telemonitoring system.
-
•
The COVID-19 emergency, its related global mobility restrictions and fear of hospitalization further highlighted the need of a comprehensive solution for patients at risk of stroke recurrence.
What this study adds
-
•
We proposed and performed a preliminary evaluation of a pragmatic e-health system to carry out multiparametric telemonitoring and to support minor stroke/TIA patients in the sub-acute phase.
-
•
The proposed solution allowed to promptly and remotely identify vital sign alterations at home during the early post-acute phase, allowing therapy and behavioral intervention adjustments.
-
•
The 3-month follow-up showed total adherence of prescribed therapy and no stroke/TIA recurrence or other emergency department admissions.
-
•
We observed a significant improvement of quality of life, as well as a significant reduction of anxiety and depression status.
-
•
The proposed e-Health solution and telemonitoring protocol may be highly useful for early post-acute remote patient management, thus supporting constant monitoring and patient adherence to the treatment pathway, especially during the COVID-19 emergency.
Declaration of Competing Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Figures are original and not previously published.
Acknowledgements
M. A., G. F. and P. P. are supported by Cloud Assisted for Health and Safety - CASSIA project (POR-FESR FVG 2014-2020). The authors would like to thank Matteo di Franza for editorial assistance and English proof-reading.
References
- 1.Parmet W.E., Sinha M.S. Covid-19—the law and limits of quarantine. N. Engl. J. Med. 2020;382(15):e28. doi: 10.1056/NEJMp2004211. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick P.B. The global burden of stroke: persistent and disabling. Lancet Neurol. 2019;18(5):417–418. doi: 10.1016/S1474-4422(19)30030-4. [DOI] [PubMed] [Google Scholar]
- 3.Naccarato M., Scali I., Olivo S., et al. Has COVID-19 played an unexpected “stroke” on the chain of survival? J. Neurol. Sci. 2020 doi: 10.1016/j.jns.2020.116889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furlanis G., Ajčević M., Scali I., et al. CT perfusion in hyper-acute ischemic stroke: the acid test for COVID-19 fear. Neuroradiology. 2021 doi: 10.1007/s00234-021-02639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zini A., Romoli M., Gentile M., et al. The stroke mothership model survived during COVID-19 era: an observational single-center study in Emilia-Romagna, Italy. Neurol. Sci. 2020;41(12):3395–3399. doi: 10.1007/s10072-020-04754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen-Huynh M.N., Tang X.N., Vinson D.R., et al. Acute stroke presentation, care, and outcomes in community hospitals in Northern California during the COVID-19 pandemic. Stroke. 2020;51(10):2918–2924. doi: 10.1161/STROKEAHA.120.031099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmanabhan N., Natarajan I., Gunston R., et al. Impact of COVID-19 on stroke admissions, treatments, and outcomes at a comprehensive stroke centre in the United Kingdom. Neurol. Sci. 2020:1–6. doi: 10.1007/s10072-020-04775-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furie K.L., Kasner S.E., Adams R.J., et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 9.Mohan K.M., Wolfe C.D.A., Rudd A.G., et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 10.Valls J., Peiro-Chamarro M., Cambray S., et al. A current estimation of the early risk of stroke after transient ischemic attack: a systematic review and meta-analysis of recent intervention studies. Cerebrovasc. Dis. 2016;43:90–98. doi: 10.1159/000452978. [DOI] [PubMed] [Google Scholar]
- 11.van Wijk I., Kappelle L.J., van Gijn J., et al. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet. 2005;365:2098–2104. doi: 10.1016/S0140-6736(05)66734-7. [DOI] [PubMed] [Google Scholar]
- 12.McManus R.J., Mant J., Bray E.P., et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomized controlled trial. Lancet. 2010;376:163–172. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 13.Omboni S., Guarda A. Impact of home blood pressure telemonitoring and blood pressure control: a meta-analysis of randomized controlled studies. Am. J. Hypertens. 2011;24:989–998. doi: 10.1038/ajh.2011.100. [DOI] [PubMed] [Google Scholar]
- 14.Boddy D., King G., Clark J., et al. The influence of context and process when implementing e-health. BMC Med. Inform. Decis. Mak. 2009;9(1):9. doi: 10.1186/1472-6947-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnett K., Mercer S.W., Norbury M., et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2011;380:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 16.Lasorsa I., D’Antrassi P., Ajčević M., et al. Personalized support for chronic conditions: a novel approach for enhancing self-management and improving lifestyle. Appl. Clin. Inform. 2016;7(3):633–645. doi: 10.4338/ACI-2016-01-RA-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furlanis G., Ajčević M., Naccarato M., et al. e-Health vs COVID-19: home patient telemonitoring to maintain TIA continuum of care. Neurol. Sci. 2020;41(8):2023–2024. doi: 10.1007/s10072-020-04524-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheli M., Dinoto A., Olivo S., et al. SARS-CoV-2 pandemic and epilepsy: the impact on emergency department attendances for seizures. Seizure. 2020;82:23–26. doi: 10.1016/j.seizure.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pham S., Yeap D., Escalera G., et al. Wearable sensor system to monitor physical activity and the physiological effects of heat exposure. Sensors. 2020;20(3):855. doi: 10.3390/s20030855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernocchi P., Vanoglio F., Baratti D., et al. Home-based telesurveillance and rehabilitation after stroke: a real-life study. Top. Stroke Rehabil. 2016;23(2):106–115. doi: 10.1080/10749357.2015.1120453. [DOI] [PubMed] [Google Scholar]
- 21.W Hanley J., Fairbrother P., Krishan A., et al. Mixed methods feasibility study for a trial of blood pressure telemonitoring for people who have had stroke/transient ischaemic attack (TIA) Trials. 2015;16:117. doi: 10.1186/s13063-015-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieber B.A., Taylor B., Appelboom G., et al. Meta-analysis of telemonitoring to improve HbA1c levels: promise for stroke survivors. J. Clin. Neurosci. 2015;22(5):807–811. doi: 10.1016/j.jocn.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Jhaveri M.M., Benjamin-Garner R., Rianon N., et al. Telemedicine-guided education on secondary stroke and fall prevention following inpatient rehabilitation for Texas patients with stroke and their caregivers: a feasibility pilot study. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers W.J., Rabinstein A.A., Ackerson T., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 25.Adams H.P., Jr., Bendixen B.H., Kappelle L.J., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Parmanto B., Lewis A.N., Jr., Graham K.M., et al. Development of the telehealth usability questionnaire (TUQ) Int. J. Telerehabil. 2016;8(1):3. doi: 10.5195/ijt.2016.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 28.Herdman M., Gudex C., Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buoite Stella A., Ajčević M., Furlanis G., et al. Smart technology for physical activity and health assessment during COVID-19 lockdown. J. Sports Med. Phys. Fitness. 2020 doi: 10.23736/S0022-4707.20.11373-2. [DOI] [PubMed] [Google Scholar]
- 30.Lewington S., Clarke R., Qizilbash N., et al. Prospective Studies Collaboration: age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 31.Katsanos A.H., Filippatou A., Manios E., et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69:171–179. doi: 10.1161/HYPERTENSIONAHA.116.08485. [DOI] [PubMed] [Google Scholar]