Abstract
Background
The aim of this study was to estimate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection rates in the small rural state of Arkansas, using SARS-CoV-2 antibody prevalence as an indicator of infection.
Methods
We collected residual serum samples from adult outpatients seen at hospitals or clinics in Arkansas for non–coronavirus disease 2019 (COVID-19)–related reasons. A total of 5804 samples were identified over 3 time periods: 15 August–5 September 2020 (time period 1), 12 September–24 October 2020 (time period 2), and 7 November–19 December 2020 (time period 3).
Results
The age-, sex-, race-, and ethnicity-standardized SARS-CoV-2 seroprevalence during each period, from 2.6% in time period 1 to 4.1% in time period 2 and 7.4% in time period 3. No statistically significant difference in seroprevalence was found based on age, sex, or residence (urban vs rural). However, we found higher seroprevalence rates in each time period for Hispanics (17.6%, 20.6%, and 23.4%, respectively) and non-Hispanic Blacks (4.8%, 5.4%, and 8.9%, respectively) relative to non-Hispanic Whites (1.1%, 2.6%, and 5.5%, respectively).
Conclusions
Our data imply that the number of Arkansas residents infected with SARS-CoV-2 rose steadily from 2.6% in August to 7.4% in December 2020. There was no statistical difference in seroprevalence between rural and urban locales. Hispanics and Blacks had higher rates of SARS-CoV-2 antibodies than Whites, indicating that SARS-CoV-2 spread disproportionately in racial and ethnic minorities during the first year of the COVID-19 pandemic.
Keywords: antibodies, COVID-19, health disparities, SARS-CoV-2, seroprevalence, temporal variation
In this prospective convenience sampling of remnant sera, we found increasing SARS-CoV-2 seroprevalence from 2.6% to 7.4% during August–December 2020. Higher seroprevalence rates were found in Hispanics and Blacks compared to Whites across all time periods.
Since emerging in 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread around the world, causing high morbidity and mortality [1–4]. SARS-CoV-2 infections in the United States (US) were initially concentrated in cities but subsequently spread to rural areas [5–7]. Determining how much of the population has been infected with SARS-CoV-2 is critical for national, state, and local officials as they consider measures to contain the virus and manage the pandemic. Limited testing in the initial stages of the pandemic, coupled with the potential for asymptomatic spread, made it difficult to determine the true prevalence of SARS-CoV-2 infections in the population. Some reports estimate that 40%–45% of cases of SARS-CoV-2 were asymptomatic [8–11]. A more effective way to quantify infection rates and include a more representative group is population-based seroprevalence surveys [5, 12, 13]. Antibodies generated in response to SARS-CoV-2 infections, even asymptomatic ones, can remain in the blood for months to years [14–16]. Consequently, determining the number of people with SARS-CoV-2–specific antibodies can serve as a surrogate for determining SARS-CoV-2 infection rates.
Arkansas is a rural state with an ethnically and racially diverse population of approximately 3.2 million. The following manuscript reports our work to prospectively compare SARS-CoV-2 seroprevalence in a convenience sample of remnant sera from subjects in urban and rural settings and across age, racial, and ethnic groups in the early stages of the pandemic from August through December 2020.
MATERIALS AND METHODS
Human Specimens
Remnant serum samples collected for routine, non–coronavirus disease 2019 (COVID-19)–related outpatient clinical laboratory tests were obtained from the University of Arkansas for Medical Sciences (UAMS) in Little Rock, Arkansas; family medicine clinics in Springdale, Fort Smith, and Pine Bluff, Arkansas; and the Arkansas Department of Health (ADH) with locations across the state. These locations were selected to provide broad geographic coverage across the state. Many of the ADH samples were obtained for evaluation of sexually transmitted infections, while the hospital/regional clinic samples had a multitude of reasons for collection. Every serum sample obtained from an ADH site and sent to the central laboratory in Little Rock was procured for this study during the appropriate time period listed below. Samples were collected across 3 time periods: 15 August–5 September 2020 (time period 1), 12 September–24 October 2020 (time period 2), and 7 November–19 December 2020 (time period 3). Time period 1 was used as a proof of concept and was shorter in duration than time periods 2 or 3. These time periods were chosen to provide ample time to obtain sample numbers and give a broad sampling across the state.
Patient Consent Statement
The study was reviewed and approved by the UAMS Institutional Review Board (IRB numbers 261232 and 260916) as an exempt study with waivers for consent and the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Patient Inclusion and Exclusion Criteria
Inclusion criteria for serum collection were age ≥18 years, Arkansas residency, and a specimen collected at one of the study sites. Samples were excluded with the following diagnosis codes: immunodeficiency (primary immunodeficiency [D80–D89], transplant recipient [all codes beginning with Z94], and cancer [C00–D49]). Samples from patients receiving chemotherapy (prior 2 months), steroids (prior 30 days), and/or intravenous immunoglobulin (prior 6 months) were also excluded. These criteria were meant to exclude potential false-positive serological testing or testing those patients with immunosuppression.
Data Collection and Storage
At UAMS, electronic health record data contained within the Arkansas Central Data Repository were examined by an honest broker according to the study inclusion criteria to identify study samples. A similar procedure was followed for ADH samples. Remnant samples were defined as clinical samples requiring no additional testing 5–7 days after a clinical visit. Remnant samples have been widely used for studies in special populations [17–19], such as premature infants where extensive blood sampling is a safety concern, or in resource-constrained environments. All samples were stored at 4°C until shipment to the research laboratory. All clinical and demographic variables were stored in a protected REDCap database [20, 21] and included patient age, sex, race, ethnicity, ZIP code, and county of residence. Urban vs rural determination was made by cross-referencing patient zip codes with the Federal Office of Rural Health Policy’s data files identifying nonmetropolitan counties and rural census tracts [22].
Laboratory Methods
SARS-CoV-2 antibody positivity was determined using a 2-step process, consistent with the US Centers for Disease Control and Prevention (CDC) guidelines [23]. Serum was inactivated at 56°C for 1 hour prior to testing. All sera were tested for SARS-CoV-2 receptor-binding domain immunoglobulin G (IgG) antibodies using the Beckman Coulter Access SARS-CoV-2 IgG (Brea, California) in the Clinical Laboratory at UAMS. The Access SARS-CoV-2 IgG assay in the UAMS Clinical Laboratory had a positive percent agreement of 74%, 94%, and 100% with reference samples collected 0–7, 8–14, and ≥15 days, respectively, after a positive SARS-CoV-2 reverse-transcription polymerase chain reaction (PCR) test. The negative percent agreement was 100% for samples collected prior to the SARS-CoV-2 pandemic.
Confirmation of specimens that scored as reactive by the Access SARS-CoV-2 IgG assay was performed using a 4-antigen confirmation test enzyme-linked immunosorbent assay (ELISA) as described previously [24]. An additional 5% of negative sera were randomly selected and tested in parallel. Approximately 44 samples in duplicate were tested on each run to diminish intrarun variability. To decrease interrun variability, negative pre–COVID-19 samples and samples with known positive SARS-CoV-2 antibodies were included in each ELISA.
Statistical Analyses
The prevalence of SARS-CoV-2 antibodies in the sample was reported with 95% confidence intervals (CIs) obtained using exact binomial distributions. Age-, sex-, and race/ethnicity-standardized prevalence rates were calculated using 2019 US Census Bureau Arkansas state adult population estimates [25]. Separate univariable and multivariable logistic regressions were employed to determine associations between variables and SARS-CoV-2 antibody positivity for each time period.
The significance of a monotone trend of the positivity to SARS-CoV-2 was tested by the Cochran-Armitage method. A multivariable logistic regression using the backward selection algorithm was employed to test the trend effect of race/ethnicity by the time period, starting with main effects and 2-way interaction terms, and in each step retaining only the factors showing significant associations (P < .2). The final model included sex, race/ethnicity, sample collection site, time period, and the 2-way interaction terms: time period with race/ethnicity and time period with the site.
Because the outcome rate was <10%, a bias-reducing penalized likelihood optimization was applied for all of the logistic regression fittings [26]. Goodness-of-fit was examined by the deviance test results, which did not indicate model-fitting concerns. Statistical significance was set at .05. All analyses were conducted using SAS (version 9.4) and graphics were created using R (version 4.0.2) and ArcGIS Pro (version 2.7.3).
RESULTS
Cohort Characteristics
Table 1 provides the demographic characteristics for each time period. We collected 1301 serum samples in time period 1, 2098 in time period 2, and 2405 in time period 3, for a total of 5804 samples. In terms of age distribution, the age groups 40–49 years and ≥70 years had the smallest number of samples (834 [14.4%] and 839 [14.5%], respectively), and the age group 18–29 years comprised the highest number of samples (1225 [21.1%]). The age distribution was generally consistent across each collection period, with a mean age of 47.8 years for time period 1, 48.6 years for time period 2, and 47.2 for time period 3. More specimens were collected from females (n = 3989 [68.8%]) than from males (n = 1808 [31.2%]). The racial and ethnic distribution of the study population was 46.9% non-Hispanic White (n = 2622), 43.2% non-Hispanic Black (n = 2415), and 6.0% Hispanic (n = 337). A total of 213 specimens (3.8%) were collected from patients who did not identify as White, Black, or Hispanic. Most specimens were collected from patients living in urban areas (n = 4954 [85.4%]) compared to rural areas (n = 844 [14.6%]). Samples were obtained from 74 of 75 counties in the state (Supplementary Figure 1).
Table 1.
Demographics of Sampled Populations in Arkansas Over 3 Collection Periods, August–December 2020
| Characteristica | Time Period 1 | Time Period 2 | Time Period 3 | Total | Arkansas |
|---|---|---|---|---|---|
| (n = 1301) | (n = 2098) | (n = 2405) | (N = 5804) | (n = 2 282 191) | |
| Age group | |||||
| 18-29 y | 257 (19.8) | 406 (19.4) | 562 (23.4) | 1225 (21.1) | 471 866 (20.7) |
| 30-39 y | 244 (18.8) | 394 (18.8) | 428 (17.8) | 1066 (18.4) | 377 230 (16.5) |
| 40-49 y | 205 (15.8) | 294 (14.0) | 335 (13.9) | 834 (14.4) | 355 760 (15.6) |
| 50-59 y | 211 (16.2) | 335 (16.0) | 358 (14.9) | 904 (15.6) | 373 048 (16.3) |
| 60-69 y | 209 (16.1) | 349 (16.6) | 377 (15.7) | 935 (16.1) | 349 101 (15.3) |
| ≥70 y | 175 (13.5) | 319 (15.2) | 345 (14.4) | 839 (14.5) | 355 186 (15.6) |
| Missing | 0 | 1 | 0 | 1 | … |
| Sex | |||||
| Female | 898 (69.3) | 1430 (68.2) | 1661 (69.1) | 3989 (68.8) | 1 176 416 (51.5) |
| Male | 398 (30.7) | 668 (31.8) | 742 (30.9) | 1808 (31.2) | 1 105 775 (48.5) |
| Missing | 5 | 0 | 2 | 7 | … |
| Race/ethnicity | |||||
| White | 565 (45.7) | 937 (47.6) | 1120 (47.0) | 2622 (46.9) | 1 733 824 (76.0) |
| Black | 558 (45.2) | 831 (42.2) | 1026 (43.1) | 2415 (43.2) | 341 540 (15.0) |
| Hispanic | 74 (6.0) | 126 (6.4) | 137 (5.8) | 337 (6.0) | 144 447 (6.3) |
| Other | 39 (3.2) | 75 (3.8) | 99 (4.2) | 213 (3.8) | 62 380 (2.7) |
| Missing | 65 | 129 | 23 | 217 | … |
| Area | |||||
| Urban | 1126 (86.8) | 1838 (87.6) | 1990 (82.8) | 4954 (85.4) | 1 816 489 (60.4)b |
| Rural | 171 (13.2) | 260 (12.4) | 413 (17.2) | 844 (14.6) | 1 189 468 (39.6)b |
| Missing | 4 | 0 | 2 | 6 | … |
| Collection site | |||||
| UAMS | 981 (75.4) | 1338 (63.8) | 1485 (61.8) | 3804 (65.5) | … |
| Pine Bluff | 266 (20.5) | 403 (19.2) | 319 (13.3) | 988 (17.0) | … |
| Fort Smith | … | 206 (9.8) | 131 (5.5) | 337 (5.8) | … |
| Springdale | 54 (4.2) | 151 (7.2) | 220 (9.2) | 425 (7.3) | … |
| ADH | … | … | 250 (10.4) | 250 (4.3) | … |
| SARS-CoV-2 test performed | |||||
| Yes | 139 (11.4) | 271 (12.9) | 437 (20.3) | 847 (15.5) | … |
| No | 1078 (88.6) | 1827 (87.1) | 1717 (79.7) | 4622 (84.5) | … |
| Missing | 84 | 0 | 251 | 335 | … |
| SARS-CoV-2 test result | |||||
| Positive | 12 (9.1) | 17 (6.3) | 8 (8.3) | 37 (7.4) | … |
| Negative | 120 (90.9) | 253 (93.7) | 89 (91.8) | 462 (92.6) | … |
| Missing | 1169 | 1828 | 2308 | 5305 | … |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: ADH, Arkansas Department of Health; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UAMS, University of Arkansas for Medical Sciences.
Missing data are not included in the analysis for a variable.
The Arkansas rural/urban population is total population (N = 3 005 957) rather than adults only.
Extrapolated Seroprevalence Estimates
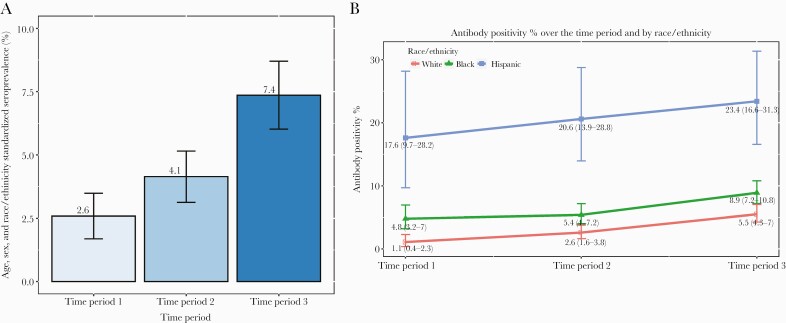
The observed seroprevalence rates were 3.8%, 4.9%, and 8.1% for time periods 1, 2, and 3, respectively (Supplementary Table 1). After standardizing by age, sex, and race/ethnicity for the Arkansas general population, the seroprevalence rate for time period 1 was 2.6% (95% CI, 1.7%–3.5%), 4.1% (95% CI, 3.1%–5.1%) for time period 2, and 7.4% (95% CI, 6.0%–8.7%) for time period 3 (Figure 1A).
Figure 1.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seropositivity rate. A, Age-, sex-, race-, and ethnicity-adjusted seroprevalence rates are shown for each time period. B, The percentage of samples with positive SARS-CoV-2 antibody tests is shown for each race/ethnicity group for each time period. Error bars indicate the 95% confidence interval.
Demographic Differences in Seroprevalence
The percentage of Hispanic patients with SARS-CoV-2 antibodies was substantially higher in all 3 time periods compared to White patients (time period 1, 17.6% vs 1.1%; time period 2, 20.6% vs 2.6%; time period 3, 23.4% vs 5.5%) (Supplementary Table 1, Figure 1B). A similar trend was observed for Black patients compared to White patients (time period 1, 4.8% vs 1.1%; time period 2, 5.4% vs 2.6%; time period 3, 8.9% vs 5.5%). Consistent with the increased percentages of SARS-CoV-2 seropositivity in Hispanic and Black patients, the adjusted odds ratios (ORs) comparing the racial and ethnic groups also were statistically significant (Table 2).
Table 2.
Adjusted Association With Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Positivity by Time Period
| Effect | Time Period 1 (n = 1301) | Time Period 2 (n = 2098) | Time Period 3 (n = 2405) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | OR (95% CI) | P Valuea | |
| Age, y | .4392 | .6390 | .8960 | |||
| 18–29 vs ≥70 | 2.04 (.62–6.65) | 1.09 (.51–2.3) | 1.14 (.64–2.04) | |||
| 30–39 vs ≥70 | 1.84 (.55–6.14) | 1.66 (.8–3.42) | 1.29 (.71–2.31) | |||
| 40–49 vs ≥70 | 1.08 (.28–4.16) | 1.13 (.51–2.54) | 0.95 (.51–1.79) | |||
| 50–59 vs ≥70 | 1.4 (.38–5.14) | 1.17 (.53–2.58) | 1.19 (.65–2.17) | |||
| 60–69 vs ≥70 | 0.71 (.16–3.09) | 1.01 (.45–2.26) | 1.21 (.67–2.21) | |||
| Sex | .3658 | .9875 | .1131 | |||
| Female vs male | 1.39 (.68–2.83) | 1 (.63–1.57) | 0.77 (.56–1.06) | |||
| Race/ethnicity | <.0001 | <.0001 | <.0001 | |||
| Black vs White | 3.9 (1.66–9.19) | 1.93 (1.14–3.26) | 2.03 (1.41–2.92) | |||
| Hispanic vs White | 15.06 (5.59–40.6) | 9.83 (5.32–18.14) | 4.42 (2.71–7.23) | |||
| Others vs White | 0.91 (.05–15.63) | 2.55 (.98–6.65) | 1.43 (.64–3.17) | |||
| Area | .5473 | .8258 | .1481 | |||
| Urban vs rural | 0.75 (.3–1.9) | 0.93 (.47–1.81) | 0.73 (.47–1.12) | |||
| Collection site | .5701 | .4906 | <.0001 | |||
| Pine Bluff vs UAMS | 1.49 (.71–3.14) | 1.49 (.87–2.54) | 0.8 (.47–1.37) | |||
| Fort Smith vs UAMS | … | 0.85 (.41–1.79) | 3.37 (1.99–5.71) | |||
| Springdale vs UAMS | 0.89 (.21–3.78) | 1.01 (.42–2.43) | 1.73 (1.01–2.97) | |||
| ADH vs UAMS | … | … | 1.75 (1.07–2.85) | |||
Bold text indicates a statistically significant difference in antibody positivity between the indicated groups.Abbreviations: ADH, Arkansas Department of Health; CI, confidence interval; OR, odds ratio; UAMS, University of Arkansas for Medical Sciences.
P value indicates whether the positivity differs between the category of a respective variable.
Over the course of the study, we observed a consistent increase in seroprevalence, including seroprevalence by racial/ethnic group. During all time periods, Blacks and Hispanics were more likely to be seropositive when adjusted for other variables (Table 3). However, a logistic regression fitting model showed that the increasing slope of seroprevalence by time period for Hispanics was significantly lower compared to the slope for Whites (OR, 0.49 [95% CI, .3–.78]) (Supplementary Table 2). Thus, although the positivity to SARS-CoV-2 was higher in Hispanics compared to Whites, the additive infection rate to Hispanics was smaller than to Whites over the time course of the study. The same finding was observed for Blacks, but there was not a statistical difference for Blacks compared to Whites (OR, 0.75 [95% CI, .5–1.12]) (Supplementary Table 2).
Table 3.
Temporal Trends of Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Seroprevalence in Relationship to Race/Ethnicity
| Characteristic | Time Period 1 | Time Period 2 | Time Period 3 | Time Period 2 vs 1 | Time Period 3 vs 2 | Trend Test |
|---|---|---|---|---|---|---|
| Positivity | Positivity | Positivity | Difference | Difference | P Value | |
| Overall | 3.77 (2.8–4.95) | 4.91 (4.02–5.92) | 8.07 (7.01–9.23) | 1.14 (–.24 to 2.53) | 3.16 (1.73–4.59) | <.0001 |
| Race/ethnicity | ||||||
| White | 1.06 (.39–2.3) | 2.56 (1.65–3.79) | 5.54 (4.27–7.04) | 1.5 (.18–2.82) | 2.97 (1.3–4.65) | <.0001 |
| Black | 4.84 (3.21–6.96) | 5.42 (3.98–7.18) | 8.87 (7.2–10.78) | 0.58 (–1.78 to 2.93) | 3.45 (1.13–5.78) | .0009 |
| Hispanic | 17.57 (9.7–28.17) | 20.63 (13.94–28.75) | 23.36 (16.56–31.34) | 3.07 (–8.12 to 14.04) | 2.72 (–7.28 to 12.73) | .3409 |
Data are presented as percentage (95% confidence interval) unless otherwise indicated.
We observed a statistically significant association with positive antibody tests for 2 age groups in 2 separate time periods (Supplementary Table 1). In time period 1, individuals aged 18–29 years were more likely to have antibodies than those aged >70 years (OR, 3.59 [95% CI, 1.12–11.52]). Individuals aged 30–39 years were more likely to have antibodies than those aged ≥70 years age group in time period 2 (crude OR, 2.17 [95% CI, 1.08–4.36]) and time period 3 (crude OR, 1.85 [95% CI, 1.00–3.06]). However, no difference in the likelihood of testing positive for SARS-CoV-2 antibodies was observed for any age group when the data was adjusted by age, sex, and area (Table 2). Together, these data indicate that age did not affect the likelihood of testing positive for SARS-CoV-2 antibodies in our study. Similarly, there was no statistically significant association between sex or area of residence (rural vs urban) and having SARS-CoV-2 antibodies during any time period.
Temporal Variations in SARS-CoV-2 Seroprevalence
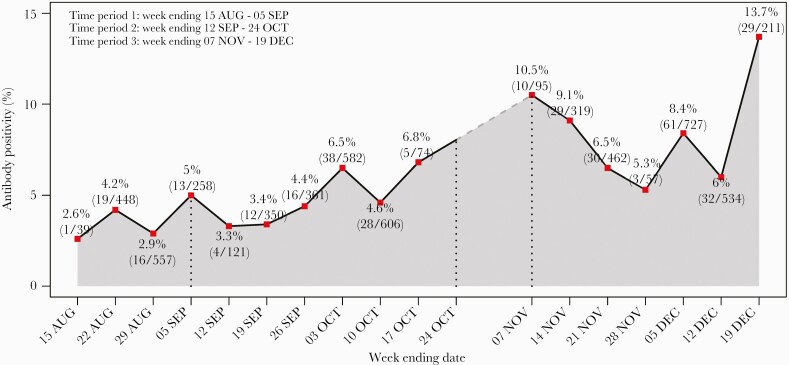
Examination of weekly changes in seroprevalence showed a gradual increase throughout the study. The peak of seroprevalence occurred in time period 3 where rates increased from 5.3% to 13.7% (Figure 2). Rates across race and ethnicity also increased accordingly with time. Seroprevalence of SARS-CoV-2 antibodies in Blacks and Hispanics were consistently higher throughout the course of the study as compared to Whites. The trend test showed that the seroprevalence significantly increased from time period 1 to time period 3 among Whites (P for trend < .0001) and Blacks (P for trend = .0009) (Table 3). Hispanics had a higher seroprevalence across 3 time periods (P for trend = .3409). In the trend of adjusted effect for race/ethnicity, the likelihood of antibody positivity for Hispanics decreased compared with non-Hispanic Whites in the later time period (OR, 0.49 [95% CI, .3–.78]) (Supplementary Table 2).
Figure 2.
Seroprevalence in Arkansas by week. There was a gradual increase in seroprevalence of severe acute respiratory syndrome coronavirus 2 antibodies over the course of the study with a peak in December 2020. Error bars indicate the 95% confidence interval.
DISCUSSION
Our study using remnant samples found that the SARS-CoV-2 seroprevalence rate in Arkansas increased from 2.6% to 7.4% from August to December 2020. During the last week of the study, the raw seroprevalence rate approached 14%. The seroprevalence we observed is consistent with both reported infections and data from American Red Cross blood donations in southern US states [27] and the CDC Multi-State Assessment of SARS-CoV-2 Seroprevalence (MASS-C) [5]. The American Red Cross blood study found only 2.9% seroprevalence from August to September in southern states, while the MASS-C data revealed 9.2% seroprevalence in December in Arkansas. It should be noted that these earlier studies had no [28] or low [5] representation from Arkansas.
For comparison, ADH reported a total of 213 267 confirmed or suspected SARS-CoV-2 infections as of 25 December 2020—roughly equivalent to 7% of the Arkansas population. Based on these numbers, our sampling potentially detected asymptomatic and untested people who were previously infected with SARS-CoV-2 in the state. Taken together, the data support the conclusion that more Arkansans had been infected with SARS-CoV-2 than previously recognized. The low seroprevalence rate in combination with slow vaccine uptake despite rapid distribution of vaccinations across the state left many people vulnerable to SARS-CoV-2 infection with variants of concern. In fact, in the spring of 2021, Arkansas experienced an uptick in cases due to the Delta variant, causing the state to rank as one of the worst for number of cases per 100 000 people during that time [29, 30].
We found higher seroprevalence of SARS-CoV-2 antibodies in Hispanics and Blacks compared to Whites throughout the study. Our data align with an earlier report that examined samples obtained from the American Red Cross for PCR testing across the US (4.11% African American, 4.35% Hispanic, and 1.65% White) [27]. In fact, CDC data from our state also noted higher rates of PCR-positive tests in Hispanics and Blacks early during our study. According to CDC data of PCR testing from the August 2020 to December 2020 time period, Hispanics had 600 incident cases per 100 000 that dropped to 388 incident cases per 100 000, while Blacks had 238 incident cases per 100 000 that increased to 385 incident cases per 100 000. In comparison, Whites had 69 incident cases per 100 000, which increased to 308 incident cases per 100 000. These numbers are consistent with our seroprevalence findings within these groups. While any attempt to explain the observed racial/ethnic disparities would be speculative, the data are consistent with a broader theme highlighting the need to understand biologic, social, and demographic factors that impact health in underrepresented minority populations.
The temporal trend of the data showed that the increase rate of infection in Whites was more than that of Hispanics or Blacks during the same time period. Hispanics and Blacks in our state were noted to have higher rates of PCR-positive tests early in the course of the time periods. Ultimately, the rates of Hispanics and Blacks who had positive PCR tests leveled over the course of our study, as shown in the previous paragraph, which explains the lower ORs and change in effect size associated with these groups.
Contrary to our expectations, SARS-CoV-2 spread uniformly across urban and rural areas of Arkansas. This finding differs from reports from the northeastern and northwestern US, and in southern cities such as Houston and New Orleans [31, 32]. However, it is worth noting that Arkansas was home to a rural super-spreader event in March 2020 [33]. These data suggest that those in rural areas of the state are just as likely to have been infected with SARS-CoV-2 as those in urban population centers.
The benefits and limitations of convenience sampling techniques have been discussed elsewhere [13]. The limitations include but are not limited to (1) the variable entry of cases from participating sites; (2) the nonrandom nature of sampling; and (3) the association of sampling with preexisting health-seeking behavior. More specific to this study, we were limited by the higher proportion of urban individuals compared to rural individuals and the higher proportion of females and Blacks compared to the Arkansas population (Table 1). It is also possible that our sampling method could favor subjects who were more ill (eg, individuals who were hospitalized) or more willing to leave their homes (eg, individuals who were evaluated in clinics). Therefore, these data do not portend representativeness of the state; rather, the data only provide the seroprevalence within this specific population. Another limitation of this study was disparate participation from some sites across the 3 time periods of the study. For example, some sites did not provide samples until time period 3. To address this limitation, logistic regressions were fitted for each time period separately and the site factor was included as an adjusted effect (Table 2). While these 2 sites (Fort Smith and ADH; Table 3) had higher odds of infection (OR, 3.37 and 1.75, respectively; Table 2), the number of samples contributed from these sites was much less than the other 3 sites, minimizing this bias.
CONCLUSIONS
Seroprevalence studies play a critical role in defining the scope of the SARS-CoV-2 pandemic. In a state with a large rural populace, our serologic analysis of remnant samples demonstrates that SARS-CoV-2 infection was more widespread than reflected by acute testing. Additionally, Hispanics and Blacks had disproportionately higher rates of SARS-CoV-2 infections. This study highlights the need to understand factors that impact health in underrepresented minority populations and the contributory role of seroprevalence in understanding the SARS-CoV-2 pandemic.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Study concept and design: K. W. B., J. C. F., J. L. K., L. J., B. A., M. W., W. N. N., V. M. C., J. S. Acquisition, analysis, or interpretation of the data: K. W. B., J. C. F., S. O., J. L. K., M. K., R. M., C. P., S. G. Y., H. H., E. O., C. K., Z. M., K. C., D. B. Draft of the manuscript: K. W. B., J. C. F., J. L. K., S. O. Critical revision of the manuscript for important intellectual content: K. W. B., J. C. F., L. J., J. L. K., S. O., J. S., B. A., M. W., N. Z., A. K., R. K. B., M. M., K. I.-C., V. M. C., P. A. M. Statistical analysis: J. J., R. D. Obtained funding: K. W. B., J. C. F., L. J., J. L. K. Administrative, technical, or material support: J. L. K., K. W. B., J. C. F. Authors J. L. K., J. C. F., and K. W. B. had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis.
Acknowledgments. The Translational Research Institute at the University of Arkansas for Medical Sciences (UAMS) provided support for clinical sample collection, sample processing, site coordination and communications, and overall implementation of this project, including provision of an honest broker, secure data storage, and data management through REDCap (National Center for Advancing Translational Sciences, National Institutes of Health 1 UL1 TR003107).
Disclaimer. The views expressed in this work are those of the authors and not necessarily those of the Arkansas Department of Health.
Financial support. This work was funded through the state of Arkansas via the Coronavirus Aid, Relief, and Economic Security Act. Other internal funds were secured from UAMS and Arkansas Children’s Research Institute.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Calderon-Larranaga A, Dekhtyar S, Vetrano DL, Bellander T, Fratiglioni L.. COVID-19: risk accumulation among biologically and socially vulnerable older populations. Ageing Res Rev 2020; 63:101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Esteve A, Permanyer I, Boertien D, Vaupel JW.. National age and coresidence patterns shape COVID-19 vulnerability. Proc Natl Acad Sci U S A 2020; 117:16118–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neumann-Podczaska A, Al-Saad SR, Karbowski LM, Chojnicki M, Tobis S, Wieczorowska-Tobis K.. COVID 19—clinical picture in the elderly population: a qualitative systematic review. Aging Dis 2020; 11:988–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID-19 and older adults: what we know. J Am Geriatr Soc 2020; 68:926–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 2021; 181:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barzin A, Schmitz JL, Rosin S, et al. SARS-CoV-2 seroprevalence among a Southern U.S. population indicates limited asymptomatic spread under physical distancing measures. mBio 2020; 11:e02426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Havers FP, Reed C, Lim T, et al. Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020 [manuscript published online ahead of print 21 July 2020]. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect 2021; 54:12–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang L, Zhang X, Zhang X, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: a prospective contact-tracing study. J Infect 2020; 80:e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oran DP, Topol EJ.. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020; 173:362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stout RL, Rigatti SJ.. Seroprevalence of SARS-CoV-2 antibodies in the US adult asymptomatic population as of September 30, 2020. JAMA Netw Open 2021; 4:e211552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shook-Sa BE, Boyce RM, Aiello AE.. Estimation without representation: early severe acute respiratory syndrome coronavirus 2 seroprevalence studies and the path forward. J Infect Dis 2020; 222:1086–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Egbert ER, Xiao S, Colantuoni E, et al. Durability of spike immunoglobin G antibodies to SARS-CoV-2 among health care workers with prior infection. JAMA Netw Open 2021; 4:e2123256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alfego D, Sullivan A, Poirier B, Williams J, Adcock D, Letovsky S.. A population-based analysis of the longevity of SARS-CoV-2 antibody seropositivity in the United States. EClinicalMedicine 2021; 36:100902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chia WN, Zhu F, Ong SWX, et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe 2021; 2:e240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen-Wolkowiez M, Ouellet D, Smith PB, et al. Population pharmacokinetics of metronidazole evaluated using scavenged samples from preterm infants. Antimicrob Agents Chemother 2012; 56:1828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen-Wolkowiez M, Benjamin DK Jr, Ross A, et al. Population pharmacokinetics of piperacillin using scavenged samples from preterm infants. Ther Drug Monit 2012; 34:312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schouwenburg S, van der Klip RFJ, Smeets TJL, et al. Review of scavenged sampling for sustainable therapeutic drug monitoring: do more with less. Ther Drug Monit 2022; 44:215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Health Resources and Services Administration. Federal Office of Rural Health Policy data files . https://www.hrsa.gov/rural-health/about-us/definition/datafiles.html. Accessed 15 February 2021.
- 23. Centers for Disease Control and Prevention. Interim guidelines for COVID-19 antibody testing. www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed 14 January 2021.
- 24. Boehme K, Kennedy JL, Snowden J, et al. Pediatric SARS-CoV-2 seroprevalence in Arkansas over the first year of the COVID-19 pandemic [manuscript published online ahead of print 16 March 2022]. J Pediatric Infect Dis Soc 2022. doi: 10.1093/jpids/piac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Census Bureau. Quick facts: Arkansas. 2021. www.census.gov/quickfacts/AR. Accessed 21 April 2021.
- 26. Firth D. Bias reduction of maximum likelihood estimates. Biometrika 1993; 80:27–38. [Google Scholar]
- 27. Dodd RY, Xu M, Stramer SL.. Change in donor characteristics and antibodies to SARS-CoV-2 in donated blood in the US, June–August 2020. JAMA 2020; 324:1677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clifton GT, Pati R, Krammer F, et al. SARS-CoV-2 infection risk among active duty military members deployed to a field hospital—New York City, April 2020. MMWR Morb Mortal Wkly Rep 2021; 70:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johns Hopkins University Center for Systems Science and Engineering. COVID-19 dashboard. https://coronavirus.jhu.edu/map.html. Accessed 27 January 2022. [DOI] [PMC free article] [PubMed]
- 30. Arkansas Department of Health. Arkansas Department of Health COVID update. 2022. https://experience.arcgis.com/experience/633006d0782b4544bd5113a314f6268a/. Accessed 27 January 2022.
- 31. Symanski E, Ensor KB, Piedra PA, et al. Population-based estimates of SARS-CoV-2 seroprevalence in Houston, TX as of September 2020 [manuscript published online ahead of print 29 April 2021]. J Infect Dis 2021. doi: 10.1093/infdis/jiab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Feehan AK, Fort D, Garcia-Diaz J, et al. Seroprevalence of SARS-CoV-2 and infection fatality ratio, Orleans and Jefferson parishes, Louisiana, USA, May 2020. Emerg Infect Dis 2020; 26:2766–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. James A, Eagle L, Phillips C, et al. High COVID-19 attack rate among attendees at events at a church—Arkansas, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:632–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.