Fig. 5. RP-6306 shows single-agent anti-tumour activity and profound tumour regressions in combination with gemcitabine.
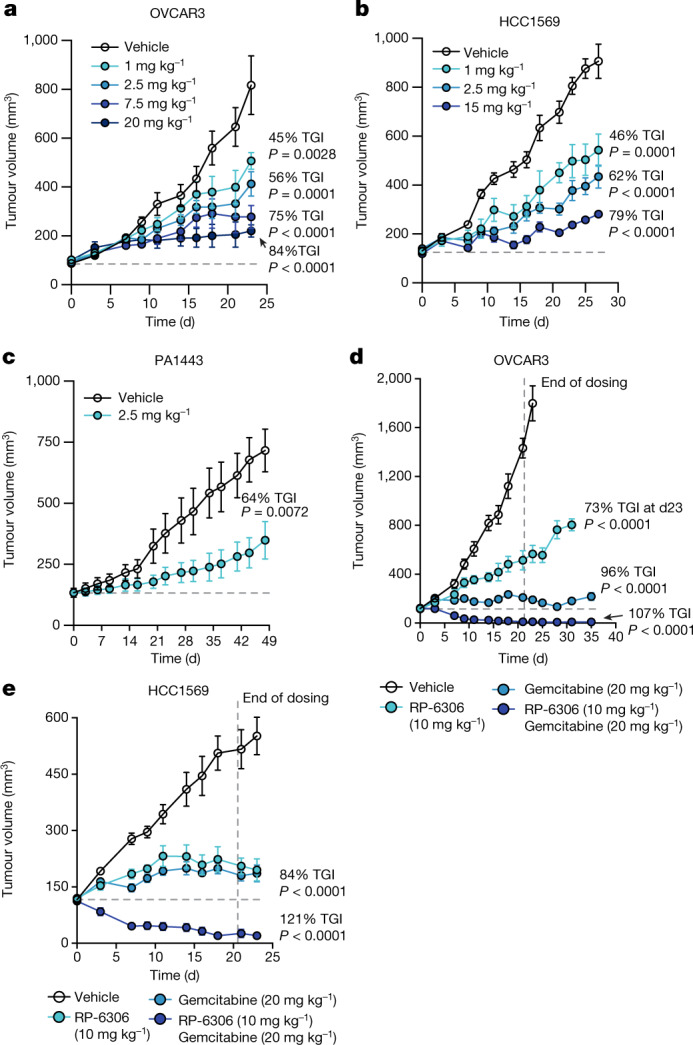
a, b, Growth of OVCAR3 (a) and HCC1569 (b) xenografts in CB-17 SCID and SCID-beige mice treated with either RP-6306 or vehicle. RP-6306 was administered orally twice daily at the indicated doses for the duration of the experiment. Results are expressed as mean tumour volume ± s.e.m. (OVCAR3 n = 8 (vehicle), 7 (1 mg kg−1), 8 (2.5 mg kg−1), 7 (7.5 mg kg−1), 8 (20 mg kg−1); HCC1569 n = 8 (vehicle),7 (1 mg kg−1), 8 (2.5 mg kg−1), 6 (15 mg kg−1)). Percentage tumour growth inhibition (% TGI) and P values relative to vehicle as determined by one-way ANOVA are shown. c, Tumour growth of a CCNE1-amplified pancreatic cancer (PA1443) patient-derived xenograft implanted in BALB/c nude mice treated either with RP-6306 or vehicle. RP-6306 was administered orally twice daily at 2.5 mg kg−1 for the duration of the experiment. Results are expressed as mean tumour volume ± s.e.m. (n = 8) with % TGI and P value relative to vehicle as determined by unpaired one-sided t-test. d, e, Tumour growth of OVCAR3 (d) and HCC1569 (e) xenografts in mice treated with either RP-6306, gemcitabine or both. Gemcitabine was administered once weekly intraperitoneally starting at day 0 and RP-6306 was given oral twice daily for 21 days after which all treatments were stopped, and tumour size was monitored for the remainder of the experiment. Results are expressed as tumour volume mean ± s.e.m. (OVCAR3 n = 7 (vehicle), 6 (10 mg kg−1 RP-6306), 7 (20 mg kg−1 gemcitabine), 7 (10 mg kg−1 RP-6306 + 20 mg kg−1 gemcitabine); HCC1569 n = 7 (vehicle), 7 (10 mg kg−1 RP-6306), 7 (20 mg kg−1 gemcitabine), 7 (10 mg kg−1 RP-6306 + 20 mg kg−1 gemcitabine)). % TGI and P values relative to vehicle as determined by one-way ANOVA are shown.
