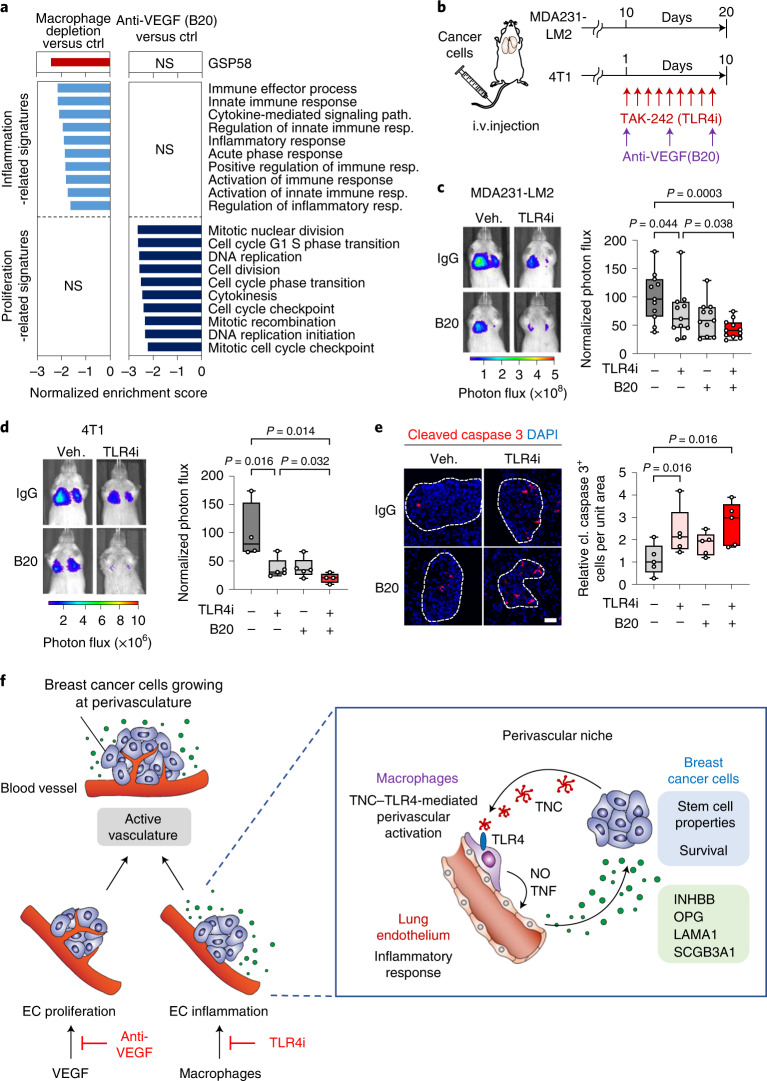
Fig. 8. TLR4 inhibition and anti-VEGF therapy target different vascular functions, with combination treatment further impeding lung metastasis.
a, GSEA of GSP58, inflammation- or proliferation-related signatures expressed in ECs from mice with either macrophage-depleted lung metastases (clodronate-liposome) or metastases treated with anti-VEGF therapy (B20). b–d, Inhibition of TLR4 and VEGF in mice harboring lung metastasis. b, Experimental outline where mice were intravenously injected with indicated breast cancer cells and treated with TLR4i or B20 as single treatments, or together as a combination treatment. c,d, Bioluminescence analysis of metastatic colonization of lungs in mice injected with MDA231-LM2 (c) or 4T1 (d) cancer cells and treated as described in b. Left, representative bioluminescence images; right, quantification of metastatic lung colonization based on bioluminescence signal. MDA231-LM2, n = 11 mice; 4T1, n = 5 mice (single TLR4i or B20 treatments) and n = 4 mice (control or double treatment). P values were calculated by one-tailed Mann–Whitney test. e, Immunofluorescence analysis of apoptosis by expression of cleaved caspase 3 in metastatic nodules treated with TLR4i, B20 or a combination of the two; n = 5 mice for each group. Scale bar, 50 μm. P values were determined by one-tailed Mann–Whitney test. c–e, Boxes show median with upper and lower quartiles, and whiskers indicate maximum and minimum values. f, Model depicting two regulatory arms of vascular activation, where VEGF promotes proliferation of ECs and macrophages, stimulated by TNC–TLR4 signaling, promote inflammatory reaction in ECs and secretion of pro-metastatic factors of the vascular niche that are utilized by cancer cells. Shown are interactions between breast cancer cells and macrophages via the TNC–TLR4 axis, which lead to macrophage activation and subsequent induction of the perivascular niche by NO and TNF to produce pro-metastatic factors including INHBB, OPG, LAMA1 and SCGB3A1. INHBB and SCGB3A1 induce stem cell properties in breast cancer cells while OPG and LAMA1 promote survival of cancer cells at the metastatic site.