Fig. 6. Stabilizing the open cryptic pocket in VP35’s IID disrupts dsRNA binding.
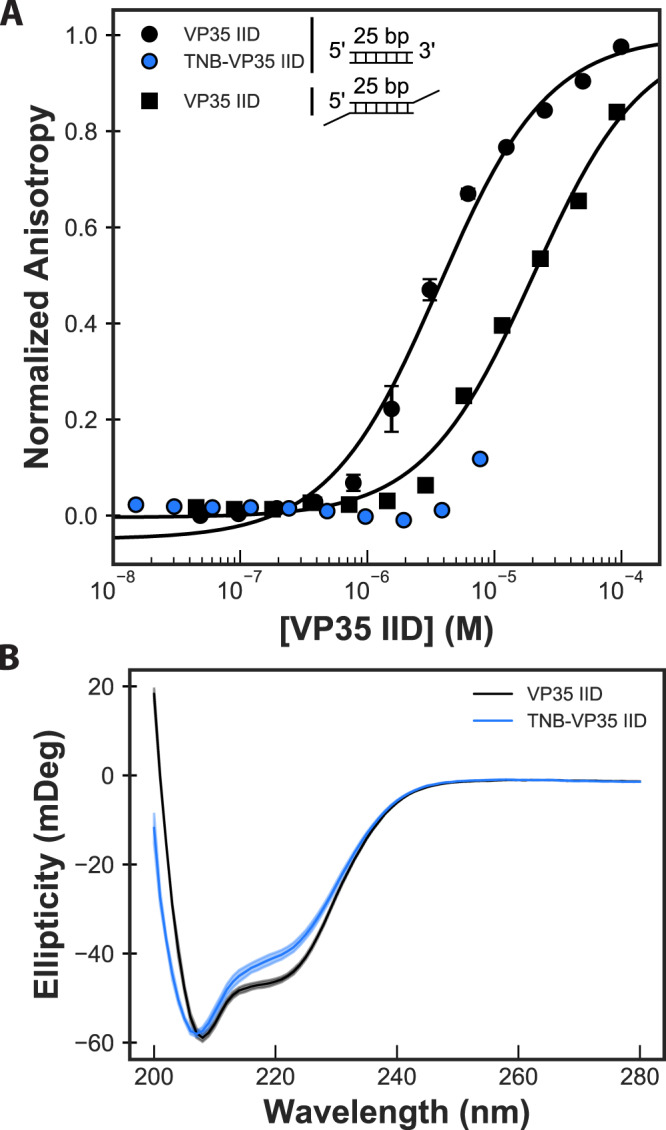
A Binding of unlabeled C247S/C275S VP35 IID to two different dsRNA constructs compared to binding of TNB-labeled protein to blunt-ended RNA. The two RNA constructs both have a 25-bp double-stranded segment, and one has 2 nucleotide overhangs on the 3’ ends. The anisotropy was measured via a fluorescence polarization assay, converted to anisotropy, fit to a single-site binding model (black lines), and normalized to the fit maximum anisotropy. The mean and standard deviation from three replicates is shown but error bars are generally smaller than the symbols. B Circular dichroism (CD) spectra of labeled and unlabeled protein demonstrate that labeling does not unfold the protein. The opaque and semi-transparent lines represent the mean and standard deviation, respectively, from three replicates. CD spectra were collected in 50 mM sodium phosphate pH 7 at 50 µg/mL protein. Source data are provided as a Source Data file.
