Abstract
Partially defatted chia flour (PDCF) is a by-product of oil extraction from chia seeds (Salvia hispanica L.). It may be used as an ingredient to improve food products, especially due to its antioxidant properties. In this work, we studied the best screw press extraction conditions that allow preserving the antioxidant properties of PDCF. A central composite design was applied to perform a response surface analysis in order to optimize the oil extraction. The variables considered for optimization were seed moisture content and pressing temperature. Besides the oil quality indicators, the study was focused on the assessment of PDCF properties, including total polyphenol content and antioxidant capacity determined by chemical methods. Our results show that, within the range of screw press conditions evaluated, the chemical quality of the oil and the antioxidant properties of PDCF are both preserved. The best results (highest oil yield and stability) were obtained under a seed moisture content of 10.2% and a pressing temperature of 58.5 °C. In general, our results indicate that screw press methodology can be applied to process chia seeds, using a wide range of conditions, to concurrently produce good quality oil and a PDCF with beneficial properties.
Keywords: Screw press, Chia seed meal, Phenolic content, Antioxidants
Introduction
Chia (Salvia hispanica L., Lamiaceae) is an ancient crop of great importance in Pre-Columbian times, originated in Central America. The current interest on these seeds is mainly due to their rich lipidic fraction (25–40%), with a high content of omega-3 polyunsaturated fatty acids (55–60%), omega-6 (18–20%), and omega-9 monounsaturated fatty acids (6–10%) (Orona-Tamayo et al. 2017).
At industrial scale, chia oil is extracted from seeds and the remaining portion (defatted chia cake) is discarded. Lately, many interesting nutritional properties have been assigned to this non-lipidic fraction, including fiber, protein content, and antioxidant properties. In the latest years, chia seeds and its derived products have been intensively studied as a functional food to improve de nutritional and technological properties in baked goods, as well as other traditional cereal- based products (Aranibar et al. 2018; Lucini Mas et al. 2020). Furthermore, the study of the functional properties of chia seeds and their by-products are being of great importance for future food applications (Fernandes et al. 2021).
Different extraction methods including cold press, solvent, and supercritical CO2, can be used to obtain chia oil. It is known that oil yield and composition are both influenced by the extraction methodology (Ixtaina et al. 2011; Fernandes et al. 2019). The selection of the method depends on the final application of the oil (i.e. food or pharmaceutical). Besides the availability of some previous studies reporting oil extraction from chia seeds with analyses focused on its composition and quality (Ixtaina et al. 2011; Bodoira et al. 2017; Fernandes et al. 2019), León-López et al. (2019) are one of the few published works that approached the study of the best conditions to optimize the polyphenol content and antioxidant activity of defatted chia seeds.
Considering the potential applications of chia by-products, the aim of this work was to determine the best screw press extraction conditions to maximize oil yield, preserving its chemical quality, and the antioxidant properties of the partially defatted chia flour (PDCF). In addition, the technological functionality of chia seeds and PDCF was evaluated to contribute to its characterization as a potential ingredient.
Materials and methods
Materials
Chia seeds (Salvia hispanica L.) were obtained in the local market in Córdoba, Argentina. The seeds were stored at 4 °C until use and brought to room temperature before analysis. All the chemical reagents and solvents were of analytical grade (Sigma Aldrich, Switzerland), including DPPH, gallic acid, Trolox, Folin-Ciocalteu and acetone. For K232 and K270 analyses, a spectrophotometric grade cyclohexane was employed (Sintorgan, Argentina).
PDCF obtention by pressing
Oil extraction was carried out with a Komet helical screw press, (IBG Monforts, Model CA 59 G, Germany). Chia seeds initial moisture content was determined following the AOCS (2009) method. Seeds were grouped in different batches (300 g) and placed into separated plastic bags, adding the calculated amount of water to reach moisture contents ranging from 9.5 to 12% wet basis (w.b). Bags were sealed and frequently rubbed in order to homogenize the moisture content throughout the sample. This was done during 48 h at room temperature without opening the bags. Subsequently, the hydrated chia seeds were heated to temperatures between 30 and 90 °C. The screw press speed (20 rpm) and the restriction dye (6 mm), as well as the minimum and maximum levels of the variables, were based on the previous study by Martínez et al. (2012).
Components of the screw press were first heated for 30 min to reach the desired temperature in each treatment. Conditioned seeds were gradually supplied to the press by gravity from the top. The temperature during the extraction process was constantly checked with a digital thermometer (TES Thermometer 1307 Type K) inserted in the restriction dye. To reach operating conditions device in regime, it was turned on 15 min before each extraction. PDCF obtained as a by-product in the oil extraction was reduced in size using a knife mill and, subsequently, sieved through a mesh with a 0.25 mm opening.
Experimental design. Optimization of the response surface extraction process
Surface response methodology (SRM) was used to assess the influence of varying conditions (moisture content and temperature) on oil yield, oil quality, and PDCF antioxidant properties. A central composite design (Montgomery 2017) was performed to identify the interaction between parameters with the greatest influence, as well as the conditions to optimize the process.
A factorial design 22 with 12 treatments (4 central points) was carried out using two different levels for the evaluated parameters: seed moisture content (SMC) (X1: 10 to 12%) and pre-pressing temperature (PT) (X2: 30 to 90 °C). The evaluated responses for extracted oils were, oil yield (OY) (Y1: g/100 g oil), fine solids content (FSC) (Y2: g solids/100 g extract), peroxide index (PI) (Y3: meq O2/kg oil), acidity index (AI) (Y4: g oleic acid/g oil), specific extinction coefficients: (Y5: K232; Y6: K270), pigment content: carotenoids (CCa) (Y7: mg/kg oil) and chlorophyll (Cl) (Y8: mg/kg oil), total tocopherols (TT) (Y9: μg tocopherols total/g oil) and antiradical capacity (ARC) (Y10: % inhibition). For the remaining non-lipidic fractions (PDCF), the evaluated responses were: total polyphenol content (TPC) (Y11: mg GAE/100 g) and antioxidant activity (AA) measured by different chemical methods: reducing power (RP), free radical scavenging capacity (AC), antiradical capacity (ARC) (Y12: mg TE/100 g PDCF). All tests and determinations were carried out at least in duplicate, randomly, and replicas of the central point were done to allow estimation of pure error as square sums according to Martínez et al. (2012).
A second-order polynomial model (Eq. 1) was used to demonstrate the responses (Yn) as function factors; where Y (dependent variable) is the estimated response, β0 represents the constant term, βi refers to the coefficients of the linear parameters, Xi are the independent variables as factors, βii refers to the coefficients of the quadratic parameter and βij refers to the coefficients of parameters of interaction.
| 1 |
Oil yield determination
Oil yield (OY) was measured considering the initial oil content in the incoming material (chia seeds, 31% dry basis, d.b.) and the remaining oil content in the cake. Both were determined according to AOCS (2009). OY is expressed as g of extracted oil/100 g of total oil present in chia seeds (Martínez et al. 2012).
Fine solids content (FSC)
FSC were determined according to Martínez et al. (2012). The screw-pressed oil samples were centrifuged at 11,000 × g/30 min. The recovered solids were washed with cyclohexane, dried and weighed. FSC is expressed as g solids/100 g extract (oil + solid).
Oil quality analysis
Peroxide index (PI), acidity index (AI) and specific extinction coefficients (K232, K270), were analyzed according to standard methods of AOCS (2009). Quantification of tocopherols (TT) was carried out using high pressure liquid chromatography (HPLC) and the antiradical capacity (ARC) was measured according to Martínez et al. (2012). Pigments (CCa; Cl) were measured spectrophotometrically according to Ixtaina et al. (2011). Both minor components were expressed as mg of pigment/kg oil.
Extraction of phenolic compounds from PDCF
The extraction of free phenolic compounds (F) was made according to our previous work Aranibar et al. (2018). Briefly, 5 g of PDCF powder were extracted with 20 mL of an acetone/water (4:1) solution during 1 h at room temperature, in darkness. The process was repeated twice. Lastly, supernatants were filtered through a cellulose filter and evaporated under reduced pressure (50 °C). Dry extracts were reconstituted with 5 mL of methanol (HPLC grade).
The solid residue obtained after free polyphenols extraction was dried at 35 °C for 2 h. To perform the extraction of the remaining portion of phenolic compounds, the bound polyphenols (B), a basic hydrolysis was carried out by adding 20 mL of 2 M NaOH solution and stirring for 15 h at 20 °C in darkness. Then, 4 mL of concentrated HCl (pH < 2) were added to stop the hydrolysis, mixing for 90 s. The sample was centrifuged at 18,500 × g for 20 min at 4 °C. The supernatant was treated with 15 mL of an ethyl ether/ethyl acetate (1:1) solution, homogenized for 1 min at 20 °C and centrifuged at 3220 × g for 10 min at 4 °C. The last supernatant was taken and filtered through a cellulose filter. This procedure was repeated 3 times to maximize the extraction of bound polyphenols. The filtered supernatants were combined and evaporated to dryness at 45 °C under reduced pressure. Finally, the dry extracts were reconstituted with 5 mL of methanol HPLC grade. All samples (F and B) were extracted in duplicate and stored at − 80 °C until analysis.
Total polyphenol content (TPC)
TPC of F and B PDCF fractions was determined by Folin-Ciocalteu method according to the procedure reported by Aranibar et al. (2018). Briefly, 1.68 mL of ultrapure-water and 100 µL of methanol were added to 5 µL of each extract and mixed for 30 s. Then, 100 µL of the Folin- Ciocalteu reagent and 300 µL of aqueous sodium carbonate (20%) were added, vortexed, and allowed to stand 120 min at room temperature in darkness. The absorbance was read at 750 nm. A linear regression was performed using gallic acid as standard for the calculation of TPC. Results are referred in mg of gallic acid equivalents (GAE) per 100 g of PDCF. All samples were analyzed in duplicate for each duplicate of extraction.
Determination of antioxidant activity of PDCF
Three chemical methods were used to measure antioxidant activity: ferric reducing ability of plasma assay (FRAP), to evaluate the reducing capacity by electrons transfer; ABTS• + radical cation decoloration assay, to assess the radical scavenging activity (TEAC); and DPPH assay, to evaluate the ability of the sample to neutralize free radicals.
FRAP assay was performed according to Benzie and Strain (1996). The working solution was prepared by combining acetate buffer pH 3.6 (3.1 g C2H3NaO2·3H2O and 16 mL C2H4O2), a 20 mM FeCl3.6H2O solution, and a 10 mM TPTZ solution in 40 mM HCl (10:1:1, respectively). Briefly, 3 mL of this solution and 95 μL of methanol were added to 5 μL of each sample. The mixtures were kept in the dark for 15 min and their absorbances measured at 593 nm. The reducing power (RP) of each sample was determined by extrapolating its absorbance on a calibration curve using Trolox as standard. The linearity range used was from 0 to 0.032 mM and the standards were processed in triplicate. Results are expressed in mmol Trolox equivalent (TE)/100 g of PDCF.
TEAC assay (Re et al. 1999), was performed using the following methodology. For the ABTS• + radical generation, 10 mL of a 7 mM ABTS solution in ultra-pure water were mixed with 6.7 mg of K2S2O8, allowing its stabilization for 16 h in darkness, at room temperature. The concentrated ABTS• + solution was diluted with methanol to an absorbance of 0.80 ± 0.02, at 734 nm at room temperature to prepare the working reagent. Three mL of working reagent were added to 5 µL of extract and 95 µL of methanol, mixed for 30 s and incubated in the dark for 30 min. The absorbance was measured at 734 nm. The radical scavenging capacity (AC) of each sample was determined by extrapolating its absorbance on a calibration curve using Trolox as standard with a linearity range of 0–0.032 mM. The samples were tested in duplicate and the results were expressed as mmol TE/100 g PDCF.
DPPH assay was carried out following the methodology of Brand-Williams et al. (1995) using a DPPH working solution in methanol (24 mg/L). Three mL of this solution were added to 5 μL of sample and 95 μL of methanol. Mixtures were kept in the dark for 15 min and measured at 515 nm. To calculate a linear regression, Trolox was also used as standard. The antioxidant capacity was analyzed in duplicate and expressed in mmol TE/100 g of PDCF.
PDCF and chia seeds functional quality
The PDCF sample associated to the highest oil yield (10.32% SMC–58.5 °C) was used to evaluate the swelling capacity (SC) following the procedure by García-Salcedo et al. (2018) Briefly, 2.5 g of sample and 30 mL of distilled water were mixed and left to settle for 24 h at room temperature. Lastly, the final volume (Vf) of samples was measured. The SC calculation was determined as follows:
| 2 |
PDCF water retention capacity (WRC) was also determined: 1 g of sample (Wi) and 30 mL of water were agitated and reserved for hydration during 18 h. Samples were centrifuged (614 × g for 30 min), the supernatant was separated and the wet residue (WR) was weighed. Afterwards, this wet residue was heated (105 °C for 24 h) and weighed again (DR) (García-Salcedo et al. 2018). Results were calculated by the following equation.
| 3 |
Water absorption capacity (WAC) was calculated according to Niba et al. (2002) as follows:
Finally, the percentage of solubility (S) of the samples was measured using the equation as follows:
| 4 |
All these properties were also calculated for raw chia seeds.
Pasting properties
PDCF viscosity properties were compared to the viscosity profile of wheat flour. Both samples were evaluated using a Rapid Viscoanalyzer (RVA 4500, Perten Instrument, Sweden) according to the official method 61–02 of the AACC (2000). Twenty-five mL of distilled water were added to 3.5 g of sample. Dispersions were stirred at 960 rpm for 10 s, and then at 160 rpm. The heating period was from 50 to 95 °C in 4.7 min, then kept at 95 °C for 2.5 min with constant stirring, and finally cooled again to 50 °C. The pasting curve was obtained with TCW3.11.298 software.
Statistical analyses
Model quality performance was assessed by ANOVA and statistical analysis of the data was performed with Statgraphic Plus software (v5.1, USA). Significant differences are reported at 0.05. A DGC comparison test was performed to reveal the paired differences between means using Infostat statistical software (Facultad de Ciencias Agropecuarias UNC Argentina) (Di Rienzo et al. 2002).
Results and discussion
Effect of processing variables on the performance and quality of the extracted oil
Table 1 shows the experimental conditions for the central composite design (comprising 12 treatments with 4 central points for pilot-scale oil extraction using the described factors and levels. Oil yield (OY) values were between 43.6 and 82.8 (g/100 g oil) and fine solids content (FSC) were between 1.88 and 3.08 (g solids/100 g extract). The main parameters were within the limits accepted by the international legislation for vegetable oils obtained by mechanical procedures (Codex 2019). The maximum peroxide value (PI) was 0.84 meq O2/kg oil and the highest acid value (AI) was 0.92 g of oleic acid/g of oil. It is important to highlight that the highest PI value obtain in our research was significantly lower than the reported by Martínez et al. (2012) (2.67 meq O2/kg of oil) and Fernandes et al. (2019) (10.98 meq O2/kg of oil), who also obtained chia oil by pressing. Moreover, all the oil samples did not present secondary oxidation, which is consistent with the low levels of PI detected. The specific extinction coefficient values (K232 and K270) were within the ranges of 1.33–2.13 and 0.25–0.30, respectively. These values were also lower than the values reported by Fernandes et al. (2019) (K232: 2.78 and K270: 0.71). As described above, the oil chemical quality was not adversely affected by the extraction process conditions.
Table 1.
Oil yield and indicators of chemical quality
| Factors | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tr | X1 | X2 | OY | FSC | K232 | K270 | PI | AI | TT | CCa | Cl | ARC |
| 1 | 9.5 | 60 | 82.8 ± 0.05f | 3.03 ± 0.19b | 1.78 ± 0.14b | 0.27 ± 0.01a | 0.42b ± 0.01b | 0.91 ± 0.02b | 372.4 ± 3.25a | 2.78 ± 0.19b | 1.31 ± 0.23b | 81.15 ± 1.13a |
| 2 | 10 | 90 | 80.9 ± 0.02e | 2.87 ± 0.12b | 1.72 ± 0.24ª | 0.28 ± 0.01a | 0.70c ± 0.08c | 0.80 ± 0.01a | 384.1 ± 5.74b | 3.43 ± 0.15c | 1.91 ± 0.22c | 81.15 ± 0.25a |
| 3 | 10 | 30 | 80.0 ± 0.21e | 2.75 ± 0.22b | 1.41 ± 0.14b | 0.30 ± 0.01a | 0.11a ± 0.00a | 0.79 ± 0.03a | 369.8 ± 3.79a | 2.63 ± 0.09b | 1.19 ± 0.09b | 80.85 ± 0.50a |
| 4 | 11 | 100 | 76.0 ± 0.15d | 2.10 ± 0.32a | 2.00 ± 0.17c | 0.30 ± 0.01a | 0.21b ± 0.00b | 0.80 ± 0.04a | 368.5 ± 3.83a | 2.26 ± 0.14b | 1.11 ± 0.11b | 81.26 ± 0.42a |
| 5 | 11 | 20 | 80.9 ± 0.03e | 1.88 ± 0.22a | 2.04 ± 0.06c | 0.27 ± 0.02a | ND | 0.90 ± 0.00b | 364.0 ± 0.85a | 2.21 ± 0.06b | 1.04 ± 0.07b | 80.61 ± 0.17a |
| 6* | 11 | 60 | 80.0 ± 0.15e | 2.22 ± 0.03a | 1.38 ± 0.03b | 0.26 ± 0.01a | 0.43b ± 0.15b | 0.80 ± 0.04a | 357.4 ± 5.77a | 2.51 ± 0.21b | 1.25 ± 0.13b | 80.50 ± 0.84a |
| 7* | 11 | 60 | 82.4 ± 0.01f | 2.13 ± 0.01a | 2.06 ± 0.01c | 0.26 ± 0.01a | 0.38b ± 0.08b | 0.88 ± 0.02b | 354.9 ± 1.12a | 2.57 ± 0.15b | 1.06 ± 0.12b | 81.44 ± 0.33a |
| 8* | 11 | 60 | 82.1 ± 0.38f | 2.17 ± 0.22a | 1.99 ± 0.08c | 0.30 ± 0.01a | 0.32b ± 0.01b | 0.90 ± 0.00b | 354.8 ± 8.18a | 2.41 ± 0.06b | 1.14 ± 0.11b | 81.74 ± 0.10a |
| 9* | 11 | 60 | 82.1 ± 0.11f | 2.40 ± 0.00a | 2.13 ± 0.04c | 0.30 ± 0.01a | 0.42b ± 0.15b | 0.92 ± 0.00b | 388.7 ± 4.24b | 2.37 ± 0.32b | 1.43 ± 0.13b | 81.26 ± 0.51a |
| 10 | 12 | 90 | 48.5 ± 0.42b | 1.99 ± 0.43a | 1.61 ± 0.01ª | 0.25 ± 0.02a | 0.84c ± 0.01c | 0.80 ± 0.01a | 360.9 ± 9.83a | 1.59 ± 0.02a | 0.60 ± 0.05a | 80.08 ± 0.25a |
| 11 | 12 | 30 | 55.6 ± 0.30c | 3.08 ± 0.29b | 1.24 ± 0.04b | 0.26 ± 0.01a | 0.26b ± 0.07b | 0.80 ± 0.03a | 354.6 ± 0.40a | 1.85 ± 0.24a | 0.68 ± 0.11a | 81.03 ± 0.08a |
| 12 | 12.5 | 60 | 43.6 ± 0.39a | 2.47 ± 0.67a | 1.33 ± 0.04b | 0.25 ± 0.02a | 0.65c ± 0.01c | 0.90 ± 0.01b | 364.0 ± 14.55a | 2.29 ± 0.56b | 0.92 ± 0.10b | 80.14 ± 0.33a |
Mean ± standard deviation (n = 2)
*Central points of the experiment
Tr: treatments; X1: SMC: % w.b. seed moisture content (%); X2: PT: pressing temperature (°C); Y1: OY: oil yield (g/100 g oil);Y2: FSC: fines solids content in oil (g solids/100 g extract); Y3: K232: conjugated dienes;Y4: K270: conjugated trienes; Y5: PI: peroxide index (meq/kg oil); Y6: AI: acidity index (g oleic acid/g oil); Y7: CCa: carotenoids (mg of carotenoids/kg oil); Y8: Cl: chlorophylls (mg of chlorophylls/kg oil); Y9: ARC: antiradical capacity (% inhibition), Y10: TT: total tocopherols (mg/kg oil)
Concerning the analyzed pigments, CCa values ranged from 1.59 to 3.43 mg/kg, and chlorophyll (Cl) values were from 0.60 to 1.91 mg/kg oil. The CCa values are above those reported by Ixtaina et al. (2011): 0.53–1.21 mg/kg, and below the values found by Bodoira et al. (2017): 5.41 mg/kg, which explains the characteristic yellow- orange color of the extracted oil samples. The differences in pigment contents observed in this study compared with the reported data, may be attributed to different geographical origins of the seeds as well as to the analytical methods used.
Regarding total tocopherols (TT), the oils obtained in the present work showed contents varying from 354.56 to 388.74 µg/g oil. These results are in the range of those informed by Ixtaina et al. (2011): 238–427 μg/g oil. However, our values were higher than the results obtained in a recent study by Abad and Shahidi (2020) who reported tocopherol concentrations of 341.06 μg/g oil. A relatively high proportion of tocopherols combined with natural antioxidants, have been shown to provide better oxidative stability in chia oil (Bodoira et al. 2017). In this case, the antioxidant activity of chia oil has ranged between 80.14 and 81.74% without significant differences between the samples. These values were also higher than those reported by Özcan et al. (2019).
SMC and PT increase plasticity of seed material and contribute to press feeding, affecting mainly OY and PI variables (Martínez et al. 2012). The statistical analysis (Figs. 1a and b) showed that only SMC had positive significant effects on oil yield (p < 0.05). Moreover, SMC between 10 and 11%, together with a PT between 40 and 60 °C, showed higher OY. Martínez et al. (2012) reported that decreasing SMC results in an increase of chia oil yield values. A comparable trend was described for other type of seeds such as moringa seeds (in the range of 8–12% SMC and 50–90 ºC), and for palm kernel oil (in the range of 3–10% SMC and 30–130 ºC) (Fakayode and Ajav 2018; Louis et al. 2020, respectively).
Fig. 1.
Effects of seed moisture content and pressing temperature on oil yield (a) and PI (b)
According to the statistical analysis, PT had positive linear significant effect on peroxide value (p < 0.05). Figure 1b shows that while PT increases, the oxidative stability decreases, resulting in higher PI values. Souza et al. (2017) revealed that temperatures above 80 °C significantly affect the chemical properties of oil and contributing to the oxidation process.
Effect of processing variables on PDCF polyphenols content and its antioxidant capacity
Table 2 shows the evaluated responses (TPC and AA) corresponding to PDCF samples from the experiments carried out. Results are shown for F and B fractions, separately. In general, TPC and AA values were higher (p < 0.05) for the F fractions of polyphenols, compared to B fractions. In accordance with our results, Rahman et al. (2017) reported lower TPC and AA values for bound polyphenols than for the free fractions of phenolic compounds of chia seeds. F-TPC values in 3 of the central points (6, 7, 9)—(11% H; 60 °C) were around the mean (186–221 mg GAE/100 g PDCF-F) without significant differences among them. Although these values were lower than those reported by Fernández-López et al. (2018) (TPC 500 mg GAE/100 g), the differences can be related to the use of ultrasonic assisted extraction by these authors.
Table 2.
Total polyphenol content (TPC) and antioxidant activity (AA) of PDCF
| Treatments | Factors | Polyphenols fraction | Responses | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDCF | |||||||||||||||
| X1 | X2 | ARC-DPPH | RP-FRAP | AC-TEAC | TPC-FOLIN | ||||||||||
| 1 | 9.5 | 60 | F | 0.47 | ± | 0.02b | 0.70 | ± | 0.03b | 0.71 | ± | 0.01b | 221.2 | ± | 5.49c |
| 2 | 10 | 90 | F | 0.44 | ± | 0.05a | 0.75 | ± | 0.03b | 0.72 | ± | 0.05b | 201.3 | ± | 3.19b |
| 3 | 10 | 30 | F | 0.42 | ± | 0.04a | 0.74 | ± | 0.04b | 0.66 | ± | 0.03b | 194.6 | ± | 14.14b |
| 4 | 11 | 100 | F | 0.39 | ± | 0.04a | 0.76 | ± | 0.04b | 0.69 | ± | 0.05b | 208.6 | ± | 9.34b |
| 5 | 11 | 20 | F | 0.38 | ± | 0.04a | 0.71 | ± | 0.03b | 0.68 | ± | 0.05b | 185.3 | ± | 18.51b |
| 6* | 11 | 60 | F | 0.46 | ± | 0.01b | 0.71 | ± | 0.06b | 0.65 | ± | 0.06b | 200.9 | ± | 7.41b |
| 7* | 11 | 60 | F | 0.39 | ± | 0.04a | 0.76 | ± | 0.05b | 0.67 | ± | 0.06b | 185.9 | ± | 13.33b |
| 8* | 11 | 60 | F | 0.49 | ± | 0.03b | 0.82 | ± | 0.07c | 0.76 | ± | 0.04c | 239.5 | ± | 12.55c |
| 9* | 11 | 60 | F | 0.43 | ± | 0.02a | 0.64 | ± | 0.04a | 0.66 | ± | 0.02b | 197.5 | ± | 10.20b |
| 10 | 12 | 90 | F | 0.40 | ± | 0.05a | 0.65 | ± | 0.06a | 0.60 | ± | 0.08a | 162.1 | ± | 21.39a |
| 12 | 12 | 30 | F | 0.37 | ± | 0.01a | 0.63 | ± | 0.04a | 0.60 | ± | 0.03a | 172.1 | ± | 15.53a |
| 11 | 12.5 | 60 | F | 0.41 | ± | 0.05a | 0.75 | ± | 0.01b | 0.66 | ± | 0.01b | 193.8 | ± | 5.92b |
| 1 | 9.5 | 60 | B | 0.09 | ± | 0.02a | 0.19 | ± | 0.06a | 0.33 | ± | 0.06a | 78.42 | ± | 14.91a |
| 2 | 10 | 90 | B | 0.07 | ± | 0.01a | 0.21 | ± | 0.03a | 0.29 | ± | 0.03a | 55.31 | ± | 0.90a |
| 3 | 10 | 30 | B | 0.06 | ± | 0.01a | 0.16 | ± | 0.04a | 0.34 | ± | 0.04a | 60.81 | ± | 5.07a |
| 4 | 11 | 100 | B | 0.08 | ± | 0.02a | 0.25 | ± | 0.06b | 0.40 | ± | 0.04b | 83.91 | ± | 18.63a |
| 5 | 11 | 20 | B | 0.09 | ± | 0.00a | 0.19 | ± | 0.03a | 0.31 | ± | 0.04a | 72.76 | ± | 9.69a |
| 6* | 11 | 60 | B | 0.08 | ± | 0.02a | 0.16 | ± | 0.09a | 0.32 | ± | 0.07a | 58.2 | ± | 15.84a |
| 7* | 11 | 60 | B | 0.09 | ± | 0.01a | 0.15 | ± | 0.01a | 0.30 | ± | 0.06a | 67.12 | ± | 3.36a |
| 8* | 11 | 60 | B | 0.10 | ± | 0.01a | 0.29 | ± | 0.05b | 0.33 | ± | 0.03a | 82.18 | ± | 12.08a |
| 9* | 11 | 60 | B | 0.09 | ± | 0.02a | 0.19 | ± | 0.05a | 0.31 | ± | 0.05a | 70.18 | ± | 19.66a |
| 10 | 12 | 90 | B | 0.12 | ± | 0.06b | 0.18 | ± | 0.04a | 0.35 | ± | 0.05a | 68.32 | ± | 10.21a |
| 11 | 12 | 30 | B | 0.05 | ± | 0.01a | 0.17 | ± | 0.05a | 0.32 | ± | 0.04a | 64.04 | ± | 4.45a |
| 12 | 12.5 | 60 | B | 0.05 | ± | 0.02a | 0.15 | ± | 0.08a | 0.30 | ± | 0.06a | 70.41 | ± | 9.30a |
Mean ± standard deviation (n = 2)
*Central points of the experiment
PDCF: partially defatted chia flour; HEX: PDCF obtention by solvent; X1: SMC: % w.b. seed moisture content (%); X2: PT: pressing temperature (°C). ARC: anti-radical capacity; RP: reducing power; AC: free radical scavenging capacity; all are expressed as mmol TE/100 g PDCF. TPC: total polyphenol content (mg GAE/100 g PDCF); n = 2. F: free; B: bound
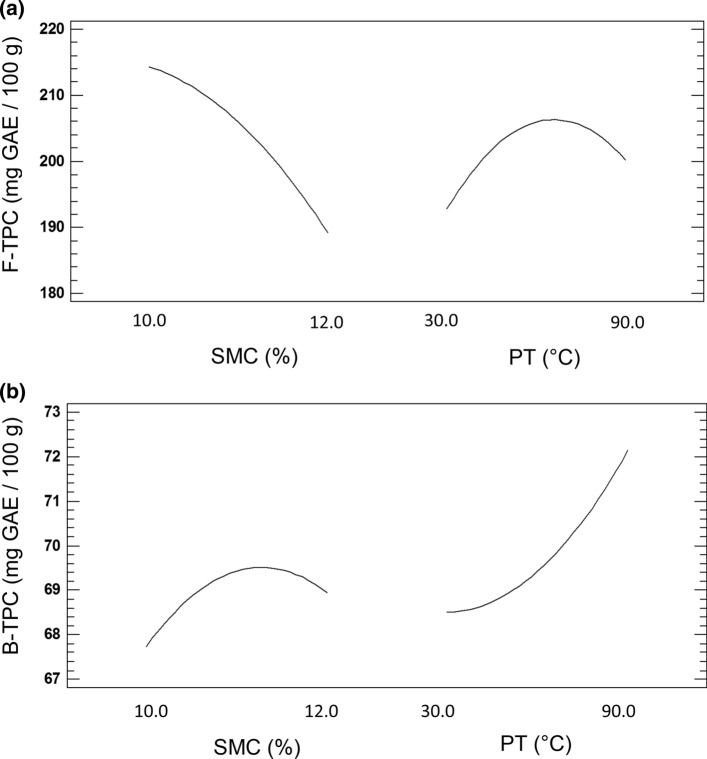
Figure 2 shows the most relevant effects on F-TPC and B-TPC. Higher values of F-TPC were observed for lower SMC values, whereas an increase of temperature to 75 °C resulted in higher F-TPC values, showing an evident decrease at temperatures above that value (Fig. 2a). On the other hand, Fig. 2b shows a completely opposed trend for the phenolic content of the B fraction. The highest B-TPC values were obtained at highest PT when seeds were hydrated at high moistures. Our results suggest that free polyphenols are less thermally stable being destroyed at temperatures above 75 °C, while polyphenols bound to the matrix are “protected” in some way, and high temperatures stimulate their release (up to 120 °C).
Fig. 2.
Main effects of oil extraction parameters on F-TPC (a) and B-TPC (b) of PDCF
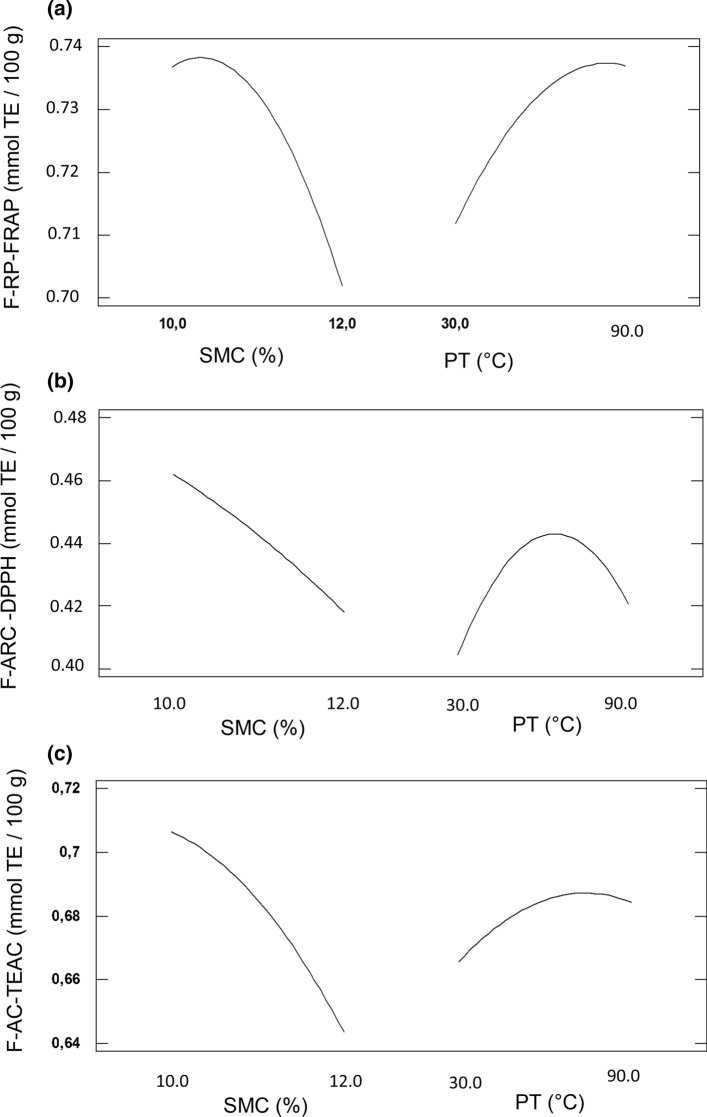
Since our findings demonstrate that the free fraction of phenolic compounds is the most abundant in chia seeds, we decided to focus our analysis on this fraction. Regarding the different indicators of AA, the highest antiradical capacity (ARC), reducing power (RP) and free radical scavenging capacity (AC) of free polyphenols, were observed for sample 8, (11% SMC, 60 °C PT), corresponding to the highest TPC value (Table 2). The AA values can be attributed to the presence of two major phenolic compounds (rosmarinic acid and its glycoside) (Pigni et al. 2020). The antioxidant capacity values varied differently depending on the method (FRAP, DPPH and TEAC) (Fig. 3). For temperatures around 80 °C, RP-FRAP and the AC-TEAC values were higher, while the highest values of ARC-DPPH were obtained at temperatures ~ 60 °C. Overall, it is known that natural antioxidants are susceptible to heat treatments (Ou et al. 2019). Nevertheless, León-López et al. (2019) have reported higher values of polyphenols and antioxidant activity in extruded PDCF at high temperatures (130 °C). Short periods of extrusion probably cause bound antioxidant compounds to be released.
Fig. 3.
Main effects on PDCF antioxidant activity. a Reduction power of free polyphenols (FRAP) b Antiradical capacity of free polyphenols (DPPH) c Free radical scavenging capacity (TEAC) of free polyphenols
PDCF and chia seeds functional quality
The ability to swell is related to the sample permeability when it comes into contact with water. A high swelling capacity (SC) is directly proportional to the protein content. PDCF and chia seeds showed SC values equal to 12.9 ± 0.14 mL/g and 13.36 ± 0.06 mL/g, respectively, with no significant differences between samples (p < 0.05). These values are in accordance to previously reported data for chia seed flour (11.82 ± 1.12 mL/g), being much higher than the SC of wheat flours (2.3 mL/g) (García-Salcedo et al. 2018). This has been attributed to the fact that chia fiber and protein contents allow an efficient water holding by forming a network that absorbs, traps and retains water as a result of swelling, increasing the volume.
PDCF water absorption capacity (WAC) (5.09 ± 0.37 g/g) compared with WAC values of chia seeds (4.89 ± 0.05 g/g) did not show significant differences (p < 0.05). These results are higher than the values reported in other types of flours, such as sweet potato, cornstarch and soybean (Julianti et al. 2017). The WAC parameter has a great influence on viscosity, volume and consistency (Niba et al. 2002). In this sense, chia proteins help to trap water tending to swell, leading to a higher water absorption and higher volumes compared with other flours. WRC values for PDCF (16.52 ± 0.37 g/g) and chia seeds (15.96 ± 0.07 g/g) did not show significant differences between them neither.
PDCF percentage of solubility (S) was significantly higher (18.91 ± 0.44%) than that found for chia seeds (7.43 ± 0.79%) (p < 0.05). There are more molecular interactions in the suspension of PDCF due to a smaller particle size. Therefore, the particles have a greater contact surface with water, improving the solubility, and facilitating the formation of a gel. Fiber and protein are the major components of chia flour (Aranibar et al. 2018), being responsible of the structural properties observed. These findings support that chia seeds and PDCF are good emulsifying agents to apply in food industry.
PDCF viscoamylographic behavior
The viscosity of flour suspensions in water during heating depends on the particular components. In the case of wheat flour (WF), it depends to a great extent on the starch content. For PDCF, it is defined by the protein and fiber content, specially due to insoluble fiber (mucilage) which allows a high water retention capacity. Understanding the behavior of these components along the heating and cooling cycles is fundamental for the development of new food products incorporating uncommon raw materials.
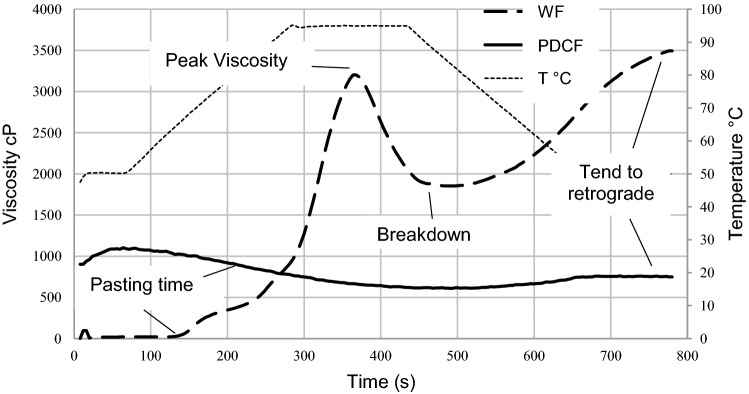
In the present work, PDCF presented a flatter and almost linear curve registering lower viscosity values compared to WF (Fig. 4). During the initial heating stage (50 to 90° C), WF showed a significantly higher viscosity peak (VP) (3210.50 cP) than the observed for PDCF (1429.6 cP). The components of WF (starch granules) are solubilized, swollen, and gelatinized, resulting in an increase of viscosity. In the case of PDCF, the solubilization of the components lead to the slight decrease of viscosity observed. During cooling (90–65 °C), WF viscosity decreased significantly to almost half of the peak value (1852.00 cP) (p < 0.05). This decrease is characteristic of entirely gelatinized wheat starch suspensions (Duda et al. 2019). The breakdown of starch granules due to the shear effect, causes the release of its compounds to the medium leading to a decrease of the viscosity. On the other hand, PDCF behavior did not necessarily depends on temperature, showing final viscosity values close to the initial viscosity (1194.67 cP) after cooling (90–50 °C). In contrast, WF final viscosity was found to be higher (3494.00 cP) than the peak value.
Fig. 4.
PDCF and wheat flour (WF) viscosity profile
PDCF components (proteins, mucilage and soluble fiber) hydrate and gel in the first 1.2 min, due to the fact that they solubilize even at lower temperatures (50 °C), maintaining a stable curve from the beginning to the end of the heating and cooling cycles. In comparison, the suspension behavior of wheat starch granules involved hydration and swelling after 6 min of starting the cycle, at a higher gelatinization temperature (66.5 °C). PDCF viscous behavior is in agreement with the results reported by García-Salcedo et al. (2018) for chia seeds flour at a concentration of 9.52% (chia flour: water, w/w). Higher concentrations (14.28% w/w) presented different behavior from the observed in PDCF. Chia seeds flour is not previously deoiled resulting in a higher lipidic content compared to PDCF. A higher level of lipids, combined with proteins, contribute to the emulsifying and stabilizing effect of the suspension, which is translated to an increase in viscosity during heating.
Conclusions
The experimental design presented here allowed us to determine an optimal combination of the processing variables for oil extraction from chia seeds by screw press, at pilot scale. Besides enhancing oil yield, we obtained a good quality oil, preserving the antioxidant properties of PDCF. Although the pressing temperature affected the oil chemical quality, all samples showed low oxidative and hydrolytic damage. The best arrangement of parameters leading to a maximum oil yield (83.7%) was 10.2% SMC and 58.5 °C PT. In general, the antioxidant capacity of PDCF was not significantly affected within the evaluated range of conditions. In addition, PDCF has shown good functional properties, such as high water absorption capacity and a good ability to gel. The main components of chia seeds (proteins and fiber) contribute to the PDCF viscous behavior, showing that an increase in temperature causes a decrease on its viscosity properties. The technological properties shown by PDCF, together with its antioxidant capacity, make this industrial residue an attractive food additive with a great potential to improve other food products. Moreover, our results indicate that a mechanical methodology, such as screw press, can be applied to obtain a good quality chia oil, together with a valuable by-product (PDCF).
Acknowledgements
This work was funded by CONICET [PIP2015-11220150100684]; FonCyT [PICT-2015-2817 and PICT 2014-2283]; SECyT, Universidad Nacional de Córdoba [30820150100160CB (2016-2018)]; Iberoamerican Project CYTED 119RT0567. C.A. had a doctoral fellowship from CONICET.
Abbreviations
- PDCF
Partially deffated chia flour
- WF
Weath flour
- SRM
Surface response methodology
- SMC
Seeds moisture content
- PT
Pressing temperature
- PI
Peroxide index
- FSC
Fine solids content
- AI
Acidity index
- CCa
Carotenoids
- Cl
Chlorophyll
- TT
Total tocopherols
- ARC
Antiradical capacity
- TPC
Total polyphenol content
- AA
Antioxidant activity
- RP
Reducing power
- AC
Free radical scavenging capacity
- OY
Oil yield
- F
Free phenolic compounds
- B
Bound polyphenols
- GAE
Gallic acid equivalent
- TEAC
Trolox equivalent antioxidant capacity
- FRAP
Ferric Antioxidant Power
- TPTZ
2,4,6‐Tri(2‐pyridyl)‐1,3,5‐triazine
- TE
Trolox equivalent
- SC
Swelling capacity
- Vf
Final volumen
- WRC
Water retention capacity
- Wi
Initial weight of sample
- WR
Wet residue
- DR
Dry residue
- WAC
Water absorption capacity
- S
Solubility
- RVA
Rapid viscoanalyzer
- DGC
Di Rienzo, Guzmán, Casanoves
Author contributions
CA was responsible for conducting the experiments (oil and PDCF obtention and analyses), analyzing the data, writing, and editing the manuscript. NBP performed the experiments (oil and PDCF obtention, PDCF analyses), analyzed the data, corrected, and edited the manuscript. MLM was in charge of the experimental design, oil quality analyses, supervision, correction and edition of the manuscript. AA, PDR and DAW contributed with the conceptualization, resources, and funding acquisition. RB was in charge of conceptualization, resources, supervision, and manuscript revision.
Funding
CONICET (PIP2015-11220150100684) Dr. Natalia B. Pigni, FonCyT (PICT-2015–2817) Dr. Daniel A. Wunderlin, Secretaria de Ciencia y Tecnología—Universidad Nacional de Córdoba (30820150100160CB (2016–2018)) Dr. Natalia B. Pigni, FonCyT (PICT-2014–2283) and Project CYTED Program (119RT0567) Dr. Marcela L. Martínez.
Availability of data and material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
Authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AACC (2000) Approved methods of the American association of cereal chemists 10th ed. St. Paul, MN, USA. Method 61–02
- Abad A, Shahidi F. Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L.) Food Prod Process Nutr. 2020;2:9. doi: 10.1186/s43014-020-00024-y. [DOI] [Google Scholar]
- AOCS (2009). Official methods and recommended practices. American oil chemists’ society. 5th ed. Methods Ce 1–62, Ce 2–66, Ce 1b-89
- Aranibar C, Pigni NB, Martinez M, Aguirre A, Ribotta P, Wunderlin D, Borneo R. Utilization of a partially-deoiled chia flour to improve the nutritional and antioxidant properties of wheat pasta. LWT Food Sci Technol. 2018;89:381–387. doi: 10.1016/j.lwt.2017.11.003. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bodoira RM, Penci MC, Ribotta PD, Martínez ML. Chia (Salvia hispanica L.) oil stability: study of the effect of natural antioxidants. LWT Food Sci Technol. 2017;75:107–113. doi: 10.1016/j.lwt.2016.08.031. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Codex A (2019) Codex standards for fats and oils from vegetable sources. Codex standard for named vegetable oils. Codex Stan 19–1981, Rev 2001–2019
- Di Rienzo J, Guzmán A, Casanoves F. A multiple-comparisons method based on the distribution of the root node distance of a binary tree. J Agric Biol Environ Stat. 2002;7:129–142. doi: 10.1198/10857110260141193. [DOI] [Google Scholar]
- Duda A, Paweł J, Dominika R, Kowalczewski PŁ. Partial wheat flour replacement with gluten-free flours in bread–quality, texture and antioxidant activity. J Microbiol Biotechnol Food Sci. 2019;9:505–509. doi: 10.15414/jmbfs.2019/20.9.3.505-509. [DOI] [Google Scholar]
- Fakayode OA, Ajav EA. Development, testing and optimization of a screw press oil expeller for moringa (Moringa oleifera) seeds. Agric Res. 2018;8:102–115. doi: 10.1007/s40003-018-0342-6. [DOI] [Google Scholar]
- Fernandes SS, Bernardino JCC, Owen PQ, Prentice C, Salas-Mellado MM, Segura-Campos MR. Effect of the use of ethanol and chia mucilage on the obtainment and techno-functional properties of chia oil nanoemulsions. J Food Process Preserv. 2021;45:e15181. [Google Scholar]
- Fernandes SS, Tonato D, Mazutti MA, de Abreu BR, da Costa CD, D’Oca P-H, Salas-Mellado MM. Yield and quality of chia oil extracted via different methods. J Food Eng. 2019;262:200–208. doi: 10.1016/j.jfoodeng.2019.06.019. [DOI] [Google Scholar]
- Fernández-López J, Lucas-González R, Viuda-Martos M, Sayas-Barberá E, Pérez-Alvarez JA. Chia oil extraction coproduct as a potential new ingredient for the food industry: chemical, physicochemical, techno-functional and antioxidant properties. Plant Foods Hum Nutr. 2018;73:130–136. doi: 10.1007/s11130-018-0670-5. [DOI] [PubMed] [Google Scholar]
- García-Salcedo ÁJ, Torres-Vargas OL, del Real A, Contreras-Jiménez B, Rodriguez-Garcia ME. Pasting, viscoelastic, and physicochemical properties of chia (Salvia hispanica L.) flour and mucilage. Food Struct. 2018;16:59–66. doi: 10.1016/j.foostr.2018.03.004. [DOI] [Google Scholar]
- Ixtaina VY, Martínez ML, Spotorno V, Mateo CM, Maestri DM, Diehl BWK, Nolasco SM, Tomás MC. Characterization of chia seed oils obtained by pressing and solvent extraction. J Food Compos Anal. 2011;24:166–174. doi: 10.1016/j.jfca.2010.08.006. [DOI] [Google Scholar]
- Julianti E, Rusmarilin H, Yusraini E. Functional and rheological properties of composite flour from sweet potato, maize, soybean and xanthan gum. J Saudi Soc Agric Sci. 2017;16:171–177. doi: 10.21315/tlsr2016.27.3.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-López L, Reyes-Moreno C, Ley-Osuna AH, Perales-Sánchez JXK, Milán-Carrillo J, Cuevas-Rodríguez EO, Gutiérrez-Dorado R. Improvement of nutritional and nutraceutical value of nixtamalized maize tortillas by addition of extruded chia flour. Biotecnia. 2019;21:56–66. doi: 10.18633/biotecnia.v21i3.1012. [DOI] [Google Scholar]
- Louis ES, Akubuo CO, Odigboh EU. Effect of some kernel factors on palm kernel oil extraction using a screw press. Agric Eng Int CIGR J. 2020;22:156–161. [Google Scholar]
- Lucini Mas A, Brigante FI, Salvucci E, Pigni NB, Martinez ML, Ribotta P, Wunderlin DA, Baroni MV. Defatted chia flour as functional ingredient in sweet cookies. How do Processing, simulated gastrointestinal digestion and colonic fermentation affect its antioxidant properties? Food Chem. 2020;316:1–9. doi: 10.1016/j.foodchem.2020.126279. [DOI] [PubMed] [Google Scholar]
- Martínez ML, Marín M, Salgado Faller CM, Revol J, Penci MC, Ribotta PD. Chia (Salvia hispanica L.) oil extraction: study of processing parameters. LWT Food Sci Technol. 2012;47:78–82. doi: 10.1016/j.lwt.2011.12.032. [DOI] [Google Scholar]
- Montgomery DC (2017) Design and analysis of experiments Wille J 9th ed. Arizona
- Niba LL, Bokanga MM, Jackson FL, Schlimme DS, Li BW. Physicochemical properties and starch granular characteristics of flour from various manihot esculenta (Cassava) Genotypes. J Food Chem Toxicol. 2002;67:1701–1705. doi: 10.1111/j.1365-2621.2002.tb08709.x. [DOI] [Google Scholar]
- Orona-Tamayo D, Valverde ME, Paredes-López O. Sustainable protein sources. 1rst. San Diego: Elsevier; 2017. Chia—The new golden seed for the 21st century; pp. 265–281. [Google Scholar]
- Ou J, Wang M, Zheng J, Ou S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019;284:90–99. doi: 10.1016/j.foodchem.2019.01.096. [DOI] [PubMed] [Google Scholar]
- Özcan MM, Al-Juhaimi FY, Mohamed Ahmed IA, Osman MA, Gassem MA. Effect of different microwave power setting on quality of chia seed oil obtained in a cold press. Food Chem. 2019;278:190–196. doi: 10.1016/j.foodchem.2018.11.048. [DOI] [PubMed] [Google Scholar]
- Pigni NB, Aranibar C, Lucini Mas A, Aguirre A, Borneo R, Wunderlin D, Baroni MV. Chemical profile and bioaccessibility of polyphenols from wheat pasta supplemented with partially-deoiled chia flour. LWT Food Sci Technol. 2020;124:109134. doi: 10.1016/j.lwt.2020.109134. [DOI] [Google Scholar]
- Rahman MJ, de Camargo AC, Shahidi F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J Funct Foods. 2017;35:622–634. doi: 10.1016/j.jff.2017.06.044. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Souza AL, Martínez FP, Ferreira SB, Kaiser CR. A complete evaluation of thermal and oxidative stability of chia oil. J Therm Anal Calorim. 2017;130:1307–1315. doi: 10.1007/s10973-017-6106-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.