Figure 3.
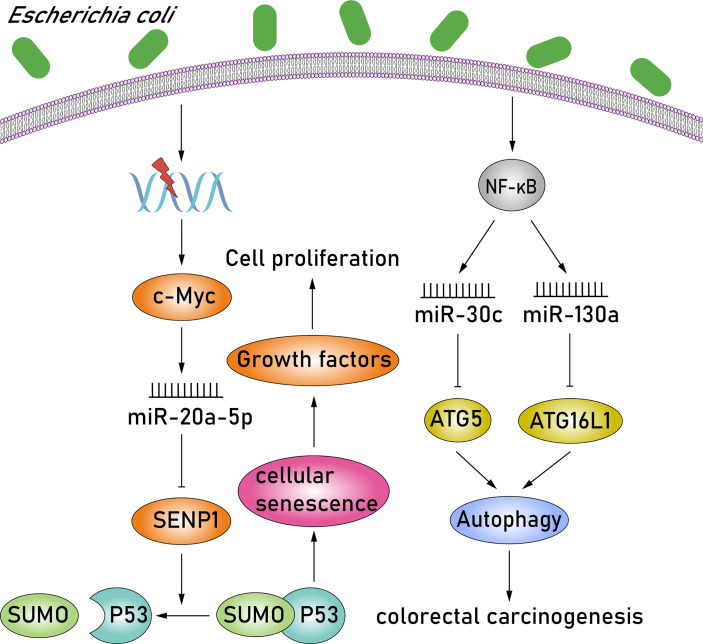
E. coli promotes cell proliferation and inflammation by modulating miR-20a-5p, miR-30c, and miR-130a. In pks+ E. coli–infected CRC cells, c-MYC is activated, and it subsequently results in the upregulation of miR-20a-5p. Upregulation of miR-20a-5p can cause the translational silencing of target SENP1. SENP1 is a key enzyme that blocks the modification of the SUMO1-conjugated p53 patterns. Moreover, the SUMOylation of p53 is identified as the key regulator of cellular senescence. The senescence of intestinal epithelial cells in pks+ E. coli–infected CRC cells consequently induces the secretion of growth factors, which play a crucial role in stimulating tumor growth. In addition, expressions of miR-30c and miR-130a were also upregulated significantly in AIEC-infected epithelial cells via activating the NF-κB pathway. Overexpression of miR-30c and miR-130a subsequently downregulates the expression of ATG5 and ATG16L1, respectively. ATG5 and ATG16L1 are members of autophagy signaling elements, and their downregulation will result in defective autophagy. Moreover, dysregulated autophagy is associated with numerous human pathologies, such as colorectal carcinogenesis.