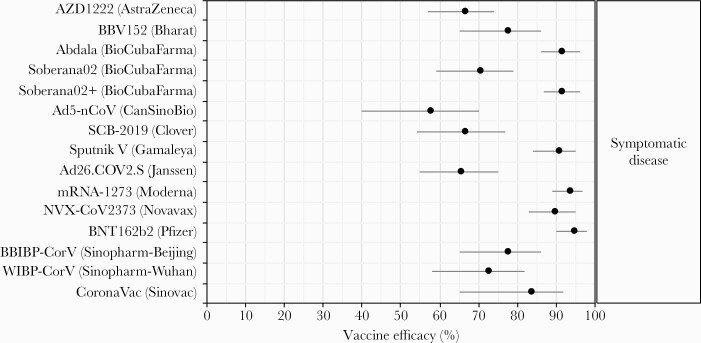
Figure 2.
Vaccine efficacy against symptomatic coronavirus disease 2019 (COVID-19), from Phase 3 clinical trials. Each efficacy value is for the complete vaccine course (1 dose for Ad26.COV2.S/Janssen and Ad5-nCoV/CanSinoBio, 3 doses for BioCubaFarma/Abdala and Soberana02+/BioCubaFarma, and 2 doses for all others). Two vaccines which withdrew from the World Health Organization Emergency Use Listing evaluation process but completed Phase 3 trials were not included (CureVac’s mRNA vaccine CVnCoV, reporting 48% efficacy [224], and Kazakhstan RIBSP’s inactivated virus vaccine QazCovid-in, reporting 82% efficacy [227]).