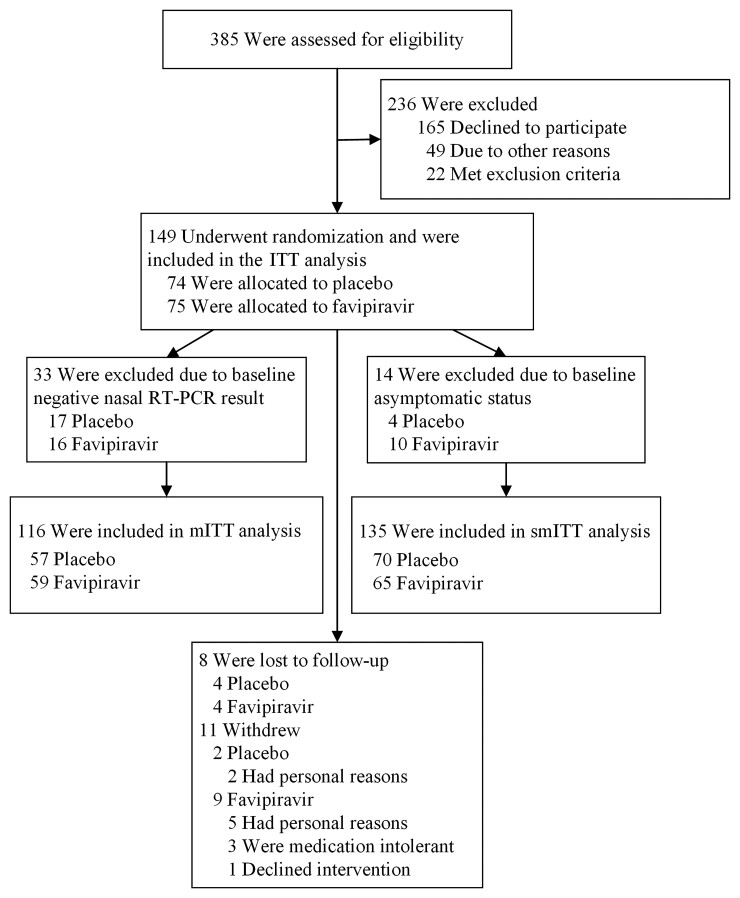
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Trial schematic showing participants screened, randomized, and followed through study completion. Two of the 3 participants randomized to receive favipiravir withdrew due to nausea and dizziness. Abbreviations: ITT, intention-to-treat; mITT, modified intention-to-treat; RT-PCR, reverse-transcription polymerase chain reaction; smITT, symptomatic modified intention-to-treat.