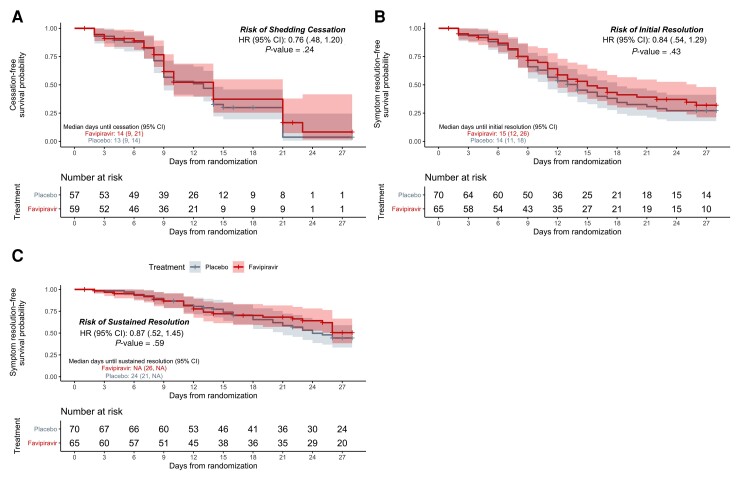
Figure 2.
Kaplan-Meier analyses of the primary and key secondary outcomes in the modified intention-to-treat population. Time until shedding cessation of SARS-CoV-2 in RT-PCR from nasal swabs (A), initial symptom resolution (B), and sustained symptom resolution (C), stratified by treatment arm: favipiravir (red) vs placebo (gray). Participants who did not experience the endpoint were censored (+ symbols) at their last positive swab for the primary outcome or at the last completed symptom questionnaire for the key secondary outcomes. Solid lines represent Kaplan-Meier survival probability; shading represents 95% confidence intervals. Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.