Figure 8.
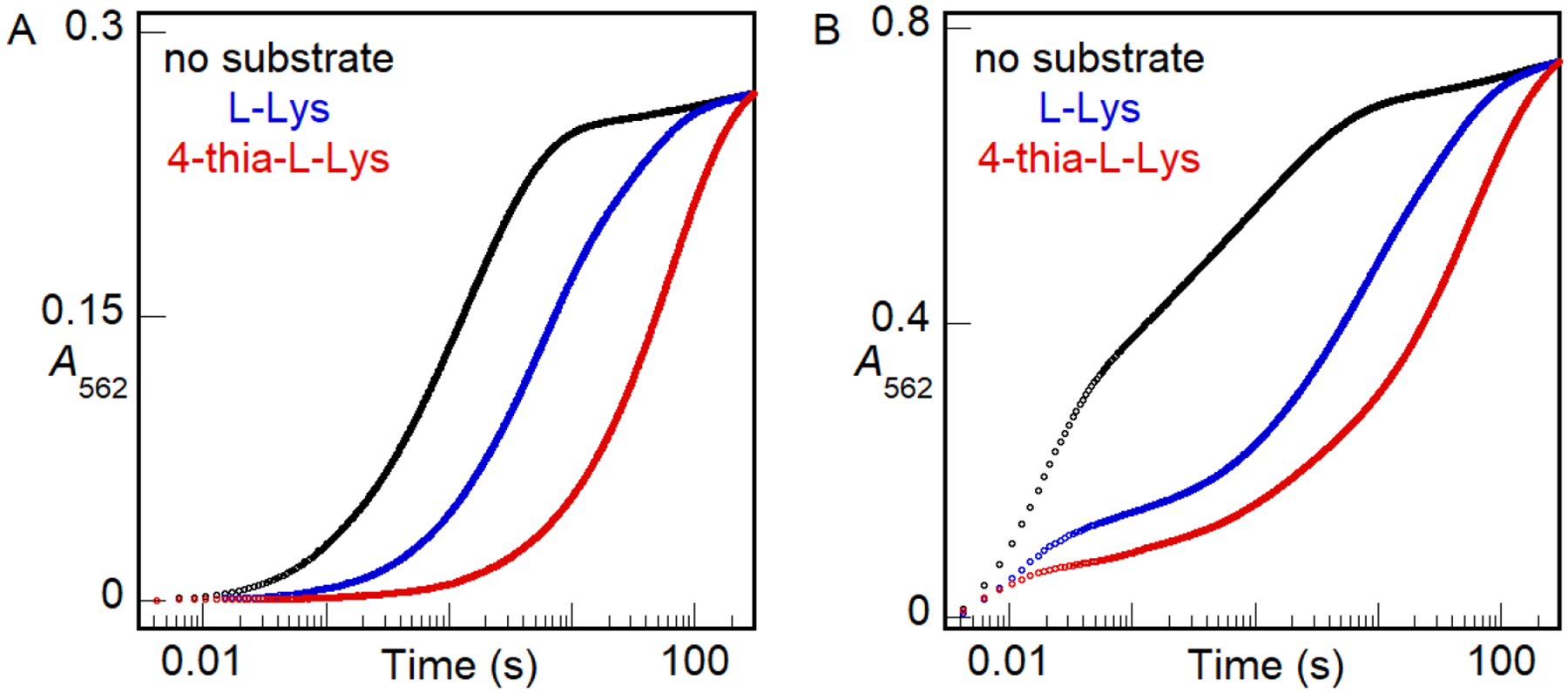
A562-versus-time traces monitoring chelation of Fe(II) by ferrozine, rapidly when free in solution or more slowly after its rate-limiting dissociation from BesC. An anoxic solution of 0.16 mM apo BesC was loaded with either (A) 0.13 mM (0.8 molar equiv) or (B) 0.32 mM (2 molar equiv) Fe(II) in the absence substrate (black) or in the presence of 12 mM L-Lys (blue) or 4-thia-L-Lys (red). This solution was subsequently mixed with an equal volume of an anoxic solution of 4 mM ferrozine. The absorbance of the purple Fe(II)•ferrozine3 complex at 562 nm (A562) was monitored as a function of reaction time. The traces shown here are representative of at least 2 trials for each condition. Results of experiments with greater Fe(II):BesC ratios in the absence of substrate are provided in Fig. S7.
