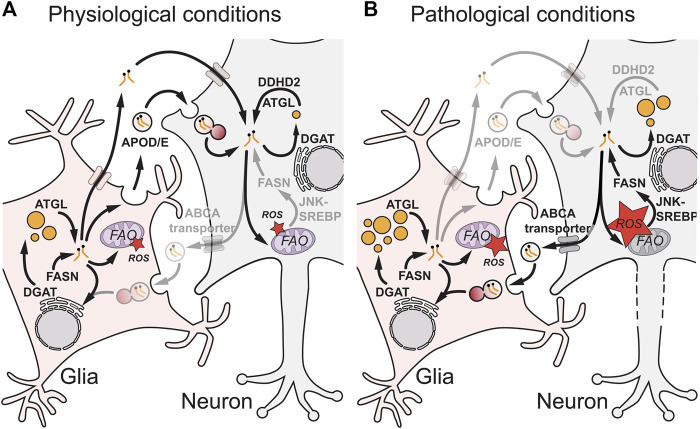
FIGURE 2.
Glial-neuronal lipid transfer during physiological and pathological conditions. During physiological conditions (A), the exchange of lipids between glia and neurons is mediated by apolipoprotein D/E (APOD/E) particles or other protein carriers such as albumin. In glia, fatty acids generated by fatty acid synthase (FASN) and converted into triacylglycerols (TAGs) via diacylglycerol acyltransferase (DGAT) can be remobilized from lipid droplets (LDs) by adipose triglyceride lipase (ATGL) for transfer to neurons or to enter mitochondria for fatty acid oxidation (FAO). In neurons, ATGL and DDHD Domain-Containing 2 (DDHD2) ensure that TAG lipolysis approximately matches TAG synthesis, preventing LD accumulation and ensuring the FA supply for neuronal functions such as membrane synthesis. Under pathological conditions (B), mitochondrial dysfunction in neurons is associated with high reactive oxygen species (ROS) that trigger c-Jun N-terminal Kinase (JNK) and sterol regulatory element-binding protein (SREBP) signalling, which increases FASN synthesis of FAs and in some circumstances leads to ectopic neuronal LDs. Excess neuronal FAs are secreted from neurons via ATP-binding cassette (ABC) A transporters and APOD/E particles, taken up by glia via endocytosis and trafficked through the endolysosomal pathway and ER via DGAT into glial LDs. Glial LDs may protect against lipotoxicity and high reactive oxygen species (ROS) via multiple non-mutually exclusive mechanisms (Figure 1B). In neurons during pathological conditions, altered TAG metabolism and ectopic LDs may contribute to dysfunction and neurodegeneration (axonal dotted line).