Abstract
Introduction
Endodontic microsurgery is a treatment of last resort for preserving natural teeth. According to radiographic evaluation, the percentage of complete healing after endodontic microsurgery is only 74.3%. The use of regenerative techniques in endodontic microsurgery for large lesions (>10 mm diameter) is therefore recommended. The most frequently used bone graft in endodontic microsurgery is anorganic bovine bone mineral (ABBM) but this only has an osteoconductive effect. Thus, when platelet-rich fibrin (PRF), a reservoir of growth factors, is used together with ABBM, it increases the regenerative effect. This study is devoted to comparing the clinical outcomes of PRF with/without ABBM as grafting biomaterials in endodontic microsurgery cases with large lesion size to provide some valuable reference data for dentists.
Methods and analysis
Sixteen patients who are in need of endodontic microsurgery will be recruited. The patients will be randomly assigned to one of two groups: an experimental group, treated with PRF/ABBM complex and collagen membrane, and a control group, treated with ABBM and collagen membrane. Clinical examination including percussion, mobility testing and presence/absence of sinus will be recorded at 7 days, and at 3, 6 and 12 months after endodontic microsurgery. A Visual Analogue Scale will be used by the patients to evaluate pain at 1, 3 and 7 days after endodontic microsurgery. Routine paralleling radiographs will be obtained before and at 3, 6 and 12 months follow-up after endodontic microsurgery. Cone-beam CT (CBCT) scans will be obtained at the 12-month follow-up. Bone formation will be evaluated according to CBCT and paralleling radiographs. The study execute time including follow-ups last from 1 June 2021 to 31 December 2024.
Ethics and dissemination
This study received approval from the Ethics Committee of Peking University School and Hospital of Stomatology. The results will be disseminated through scientific journals.
Trial registration number
Research data will be registered with the International Clinical Trials Registry Platform (ICTRP), ID: ChiCTR2100046684.
Keywords: Clinical trials, ORAL MEDICINE, ORAL & MAXILLOFACIAL SURGERY
Strengths and limitations of this study.
This trial is designed as a randomised, double-blind clinical trial.
The trial will be the first clinical trial with a novel design to compare the clinical and radiographic effects of platelet-rich fibrin/anorganic bovine bone mineral (ABBM) complex and ABBM on large periapical lesions (>10 mm diameter) after endodontic microsurgery.
This study will collect longitudinal data on patients during the subsequent 12-month follow-up.
The patients with systemic disease will not be included in this research.
Introduction
With developments in equipment, instruments and biomaterials, endodontic microsurgery has become available as a treatment of last resort for cases which are no longer suitable for retreatment.1 It is generally accepted that radiographic outcomes can be classified into complete healing, incomplete healing, uncertain healing and unsatisfactory healing.2 3 Complete bone repair is the ideal therapeutic outcome of endodontic microsurgery, however, the percentage of complete radiographic healing after endodontic microsurgery is only 74.3%.4 Thus, it is imperative to promote healing of periapical bone defects more effectively. The results of endodontic microsurgery can be influenced by several factors, especially lesion size.5 6 During the healing process, epithelial cells repopulate the wound at the highest rate which results in scar formation.7 Once complete osseous regeneration of a defect cannot occur, the defect will be filled by fibrous connective tissue.8 It has been demonstrated that 26% of defects radiographically larger than 10 mm result in scar formation after endodontic microsurgery.9 Thus, usage of grafting materials and membrane in cases with large lesion size (>10 mm diameter) is recommended.5 6 10
There are four types of bone grafts: autogenous grafts, allogeneic grafts, xenogeneic grafts and alloplastic materials. Autogenous grafts and allogeneic grafts are osteoinductive materials, while xenogeneic grafts and alloplastic materials are osteoconductive materials due to their lack of growth factors.11 It has been reported that a combination of osteoconductive materials (eg, anorganic bovine bone mineral (ABBM)) and growth factors results in faster and better healing of bone in endodontic microsurgery than osteoconductive materials only.12 13
PRF (platelet-rich fibrin) contains platelets, leucocytes and more than 100 types of growth factors including platelet-derived growth factor, transforming growth factor-beta 1, vascular endothelial growth factor and bone morphogenetic protein 2 (BMP-2), which promote the proliferation and differentiation of osteoblasts.14 Moreover, the presence of leucocytes is helpful for their anti-infection and immunomodulatory effects.15–17 Previously, PRF has been extensively used in dentistry, including the healing of extraction sockets,18 ridge preservation,19 maxillary sinus augmentation and the regeneration of periodontal lesions.20–24 It promotes the healing of soft tissue, and reduces postoperative pain, swelling and the incidence of alveolar osteitis after the extraction of impacted mandibular third molars.18 Compared with natural healing, horizontal and vertical dimension ridge preservation is more effective with the use of PRF.19 The combination of PRF and deproteinised bovine bone mineral has been shown to increase bone formation in maxillary sinus augmentation compared with deproteinised bovine bone mineral alone.20 Plenty of studies have indicated that combinations of PRF and bone grafting materials enhance periodontal regeneration in periodontal intrabony defects.21–24 In a word, PRF has the potential for osseous regeneration and healing of soft tissue, especially when combined with various bone grafting materials.25 It was also indicated that PRF reduces postoperative pain and infections due to the improvements in soft-tissue healing and the presence of microbial-fighting immune cells.26 Hitherto, there have only been two case reports in which PRF was used together with osteoconductive bone grafts in periapical surgery in order to achieve a better healing outcome.27 28 With the exception of these two case reports, there has been no clinical research into the effects of PRF in periapical surgery.
We hypothesised that PRF combined with ABBM and collagen membrane is more effective than using only ABBM and collagen membrane in endodontic microsurgery. The hypothesis will be tested in the present double-masked randomised controlled clinical trial, the design of which is described below.
Methods and analysis
The study is a prospective, single-centre randomised controlled trial. It had been approved by the Ethics Committee of Peking University School and Hospital of Stomatology (PKUSSIRB-202059179). Research procedures including assessments, interventions and follow-ups will be carried out in Peking University School and Hospital of Stomatology (Beijing, China). The main objective of this randomised controlled clinical trial is to compare and evaluate the clinical effects of PRF with or without combination with ABBM in endodontic microsurgery cases with large lesion size (>10 mm diameter). The primary hypothesis is that healing of periapical lesions will be better in the PRF/ABBM complex group.
Inclusion criteria
Patients attending the Department of Cariology and Endodontology for routine planned endodontic microsurgery will be evaluated for inclusion in this clinical trial. The age of patients to be recruited will range from 18 to 65 years. Patients will have received periodontal treatment before endodontic microsurgery. Only single-rooted teeth will be included in the study, and the periapical lesions should be classified as large periapical lesions (>10 mm diameter) according to cone-beam CT (CBCT) evaluation.
Exclusion criteria
Smokers.
Pregnant women.
Patients with systemic diseases.
Resurgery.
Unqualified coronal restoration.
Teeth with deep periodontal pockets (probing depth ≥5 mm).
Recruitment
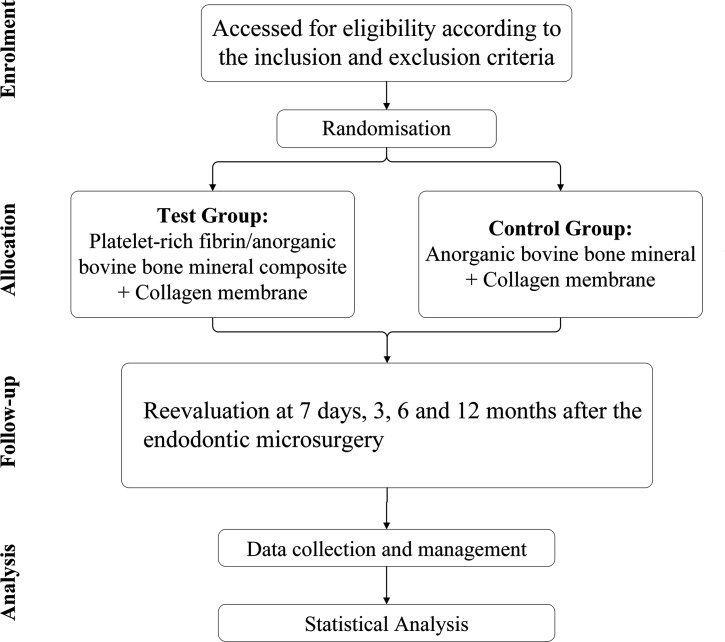
Patients who are willing to participate in this study will be recruited from the Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology. The necessity of endodontic microsurgery and collecting blood for PRF/ABBM complex will be explained to the participants. A signed informed consent form will be obtained and preserved confidentially in the cabinet. The procedures of this clinical trial are shown in figure 1.
Figure 1.
The flow chart of this clinical research.
Groups, randomisation and blinding
An experienced endodontist will perform the examination, diagnosis and assessment procedures after clinical and radiographic examinations. The sequence and allocation will be performed by a professor alone with opaque envelopes. The bone grafting material of the experimental group will be a PRF/ABBM composite, while the control group will receive ABBM only.
Interventions
All enrolled patients will be randomly divided into two groups after examination. The endodontic microsurgery will be performed by the same experienced endodontist using an endodontic microscope (F40, Leica Microsystems, Wetzlar, Germany). The procedures and principles of the endodontic microsurgeries will follow the guidelines.29 A vertical and a horizontal incision will be used to reflect the flap. Periradicular curettage will be performed after reaching the periapical lesion. After 3 mm of the root apex is resected, retropreparation and retrofilling with iRoot BP (Innovative BioCeramix, Burnaby, BC, Canada) will be performed. In the experimental group, after periradicular curettage, whole blood (10 mL) will be collected in sterile glass-coated centrifugal tube without any anticoagulant and centrifuged at 960 rpm (Medical centrifuge Auto V1, JM Instrument, Beijing, China) for 2.8 min. Then the buffy coat layer, the plasma layer and 1 to 1.5 mm of the red blood cell layer below the buffy coat layer will be harvested as the liquid PRF. Finally, ABBM (Heal-all, ZH-Bio, Yantai, China) will be mixed with the liquid PRF evenly. ABBM is a category of xenograft which has only an osteoconductive effect. The periapical defects will be filled with PRF/ABBM complex in the experimental group while the periapical defects will be filled with ABBM only in the control group. An absorbable collagen membrane (Heal-all, ZH-Bio, Yantai, China) will be applied in both groups, then the flap will be repositioned with 6–0 sutures. Amoxicillin and 0.2% chlorhexidine gluconate rinse will be prescribed to prevent postoperative infection. Sutures will be removed 5 days after the endodontic microsurgery.
Examination
Baseline examination
Before treatment, all participants will be examined carefully by a calibrated examiner. Clinical examination including percussion and mobility testing and presence/absence of sinus will be recorded simultaneously. Preoperative paralleling radiographs and CBCT scans will be obtained for each participant.
Examination during the follow-up period
Clinical examination including percussion, mobility testing and presence/absence of sinus will be performed at 7 days, 3 months, 6 months and 12 months after the endodontic microsurgery. In addition, a Visual Analogue Scale (VAS) will be recorded at 1 day, 3 days and 7 days after the endodontic microsurgery for the evaluation of postoperative pain. Paralleling radiographs will be obtained at 3 months, 6 months and 12 months. CBCT scans will be performed at the 12-month follow-up. The grouping and the treatment plan will be confidential to the examiners. The primary parameter of the present clinical trial is radiographic bone regeneration in the area of the periapical defect. The paralleling radiographs and CBCT scans will be evaluated both before and after microsurgery. Bone regeneration in periapical osseous defects will be evaluated according to the radiopacity scoring scale of paralleling radiographs by three independent blinded and calibrated examiners at the 3-month, 6-month and 12-month follow-ups after the microsurgery and compared with the paralleling radiograph after microsurgery immediately. According to a previous study,30 the change in the volume of the periapical defect is the evaluation index of the radiopacity scoring scale. CBCT data before and 12 months after microsurgery will be processed using medical image processing software (MIMICS, Materialise, Leuven, Belgium) to evaluate the changes of volume and density of the periapical defect. The data of the clinical trial will be input and kept in a specific computer and a locked cabinet by two designated member of staffs. The secondary parameters of this clinical trial include percussion, mobility testing and presence/absence of sinus. The VAS scores are also secondary parameters. All the data will be recorded and imputed in the computer simultaneously. Due to lack of data monitoring committee in our hospital, the data will be kept in the cabinet by two different researchers to make sure the accuracy and completeness of the data. An independent inspector will review the incoming data every 3 months. There will be no harms caused by the trial.
Sample size
The sample size of this clinical trial is determined by the following formula:
N1=N2=2
According to the data of a published clinical trial, which is about bone grafting in periapical osseous defects,30 the σ/δ is around 0.49. The inspection level (α) is set to 0.05, and the power is set to 0.9. For bilateral tests, the required sample size in each group is six. Considering a missed follow-up rate of 20%, the sample size should be 7.2. Consequently 16 participants will be needed. Once more than 20% participants withdrawal occur, new participants will be enrolled.
Statistical analysis
Data will be analysed using SPSS software V25.0. Statistical significance will be accepted for p values lower than 0.05. Normality and variance equality will be analysed using the Shapiro-Wilk test and the Levene variance homogeneity test, respectively. Normally distributed data will be shown as mean±SD, while non-normally distributed data will be shown as median (lower to upper quartile). Student’s t-test will be used to compare the difference between the two groups for the data with both normality and variance equality. Otherwise, the Mann-Whitney U test will be used.
Withdrawal
The patients have the right to withdraw from this clinical trial without any reason at any point during the treatment. Follow-up treatment will not be affected by the withdrawal.
Dissemination of results
The results of this clinical trial will be registered at the International Clinical Trials Registry Platform. In addition, the results will be published in a peer-reviewed journal.
Patient and public involvement
Neither patients nor the public were involved in the design, recruitment, assessment, conduct and reporting of this research. The results will be disseminated through scientific journals.
Discussion
When PRF is used together with ABBM, it increases the regenerative effect of osseous tissue,31 32 because ABBM only has an osteoconductive effect and osteoinduction is induced due to the growth factors in PRF. In a histological study, bone regenerative effects of PRF-TCP, rhBMP-2-coated TCP and TCP alone were compared, and the results showed that PRF-TCP resulted in more rapid bone healing compared with the other two groups.12 The antibacterial and anti-inflammatory effects of PRF also promoted tissue healing.14 Although PRF has many advantages, traditional PRF is not liquid, and it is challenging to mix PRF and ABBM evenly. Thus, in previous studies,33–35 the gelatinous PRF had to be minced and then mixed with ABBM, which is time-consuming. Exposing alveolar bone to air could result in bone resorption, and a long duration of surgery is not favourable.36 Therefore, our group developed a new technique to obtain a type of PRF/ABBM complex and thus avoid the need to mince the PRF.23 24 Additionally, PRF/ABBM complex as a whole had better applicability than granular ABBM, and could significantly reduce the time required for bone grafting.24 The complex together with a collagen membrane has been demonstrated to result in enhanced gains in attachment level compared with ABBM alone with a collagen membrane, indicating improved periodontal regeneration.24
To date, there is still a lack of clinical trials investigating the osseous regenerative effect of PRF/bone grafting material composites in endodontic microsurgery. This randomised controlled trial is devoted to the evaluation of the clinical outcomes of the application of a PRF/ABBM complex as a grafting biomaterial in endodontic microsurgery cases with large lesion size, in the hope of providing some scientific evidence to support endodontic microsurgeries for dentists.
Ethics and dissemination
Ethical approval had been approved by the Ethics Committee of Peking University School and Hospital of Stomatology (PKUSSIRB-202059179). The data of the clinical trial will be input and kept in a specific computer and a locked cabinet by two designated member of staffs. The results will be published in a peer-reviewed journal.
Supplementary Material
Footnotes
Contributors: The study concept was proposed by ZW, KL, ZC and BH. The manuscript was drafted by BH and KL. CZ and ZZ revised the part on randomisation. YW and YL calculated the sample size. ZW, XW and KL revised the manuscript finally. All authors have agreed with the final version of the manuscript.
Funding: This work was supported by grants from the Programme for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-17B02, PKUSSNCT-20A04 and PKUSSNCT-20B13).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Monaghan L, Jadun S, Darcey J. Endodontic microsurgery. Part one: diagnosis, patient selection and prognoses. Br Dent J 2019;226:940–8. 10.1038/s41415-019-0415-3 [DOI] [PubMed] [Google Scholar]
- 2.Rud J, Andreasen JO, Jensen JE. Radiographic criteria for the assessment of healing after endodontic surgery. Int J Oral Surg 1972;1:195–214. 10.1016/S0300-9785(72)80013-9 [DOI] [PubMed] [Google Scholar]
- 3.Molven O, Halse A, Grung B. Observer strategy and the radiographic classification of healing after endodontic surgery. Int J Oral Maxillofac Surg 1987;16:432–9. 10.1016/S0901-5027(87)80080-2 [DOI] [PubMed] [Google Scholar]
- 4.Wang Z-H, Zhang M-M, Wang J, et al. Outcomes of endodontic microsurgery using a microscope and mineral trioxide aggregate: a prospective cohort study. J Endod 2017;43:694–8. 10.1016/j.joen.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 5.Deng Y, Zhu X, Yang J, et al. The effect of regeneration techniques on periapical surgery with different protocols for different lesion types: a meta-analysis. J Oral Maxillofac Surg 2016;74:239–46. 10.1016/j.joms.2015.10.007 [DOI] [PubMed] [Google Scholar]
- 6.Sánchez-Torres A, Sánchez-Garcés Ma Ángeles, Gay-Escoda C. Materials and prognostic factors of bone regeneration in periapical surgery: a systematic review. Med Oral Patol Oral Cir Bucal 2014;19:e419–25. 10.4317/medoral.19453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobón SI, Arismendi JA, Marín ML, et al. Comparison between a conventional technique and two bone regeneration techniques in periradicular surgery. Int Endod J 2002;35:635–41. 10.1046/j.1365-2591.2002.00523.x [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedy V, Chaturvedy S. Regenerative therapy as an adjunct to periapical surgery: a case report. Int J Clin Pediatr Dent 2012;5:75–7. 10.5005/jp-journals-10005-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Kratchman S. Microsurgery in Endodontics. Wiley-Blackwell, 2018. [Google Scholar]
- 10.Lin Y-C, Lee Y-Y, Ho Y-C, et al. Treatment of large apical lesions with mucosal fenestration: a clinical study with long-term evaluation. J Endod 2015;41:563–7. 10.1016/j.joen.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 11.Chen F-M, Liu X. Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 2016;53:86–168. 10.1016/j.progpolymsci.2015.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim B-J, Kwon T-K, Baek H-S, et al. A comparative study of the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113:583–92. 10.1016/j.tripleo.2011.04.029 [DOI] [PubMed] [Google Scholar]
- 13.Taschieri S, Rosano G, Weinstein T, et al. Treatment of through-and-through bone lesion using autologous growth factors and xenogeneic bone graft: a case report. Oral Maxillofac Surg 2012;16:57–64. 10.1007/s10006-010-0251-8 [DOI] [PubMed] [Google Scholar]
- 14.Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig 2017;21:1913–27. 10.1007/s00784-017-2133-z [DOI] [PubMed] [Google Scholar]
- 15.Bielecki T, Dohan Ehrenfest DM, Everts PA, et al. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol 2012;13:1153–62. 10.2174/138920112800624373 [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Yin C, Zhao Q, et al. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J Biomed Mater Res A 2020;108:61–8. 10.1002/jbm.a.36792 [DOI] [PubMed] [Google Scholar]
- 17.Jasmine S, A T, Janarthanan K, et al. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin-a second-generation platelet concentrate-against biofilm producing oral Staphylococcus isolates. Saudi J Biol Sci 2020;27:41–6. 10.1016/j.sjbs.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daugela P, Grimuta V, Sakavicius D, et al. Influence of leukocyte- and platelet-rich fibrin (L-PRF) on the outcomes of impacted mandibular third molar removal surgery: a split-mouth randomized clinical trial. Quintessence Int 2018;49:377–88. 10.3290/j.qi.a40113 [DOI] [PubMed] [Google Scholar]
- 19.Temmerman A, Vandessel J, Castro A, et al. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: a split-mouth, randomized, controlled clinical trial. J Clin Periodontol 2016;43:990–9. 10.1111/jcpe.12612 [DOI] [PubMed] [Google Scholar]
- 20.Pichotano EC, de Molon RS, de Souza RV, et al. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: a randomized clinical trial. Clin Implant Dent Relat Res 2019;21:253–62. 10.1111/cid.12713 [DOI] [PubMed] [Google Scholar]
- 21.Bodhare GH, Kolte AP, Kolte RA, et al. Clinical and radiographic evaluation and comparison of bioactive bone alloplast morsels when used alone and in combination with platelet-rich fibrin in the treatment of periodontal intrabony defects-A randomized controlled trial. J Periodontol 2019;90:584–94. 10.1002/JPER.18-0416 [DOI] [PubMed] [Google Scholar]
- 22.Pradeep AR, Bajaj P, Rao NS, et al. Platelet-Rich fibrin combined with a porous hydroxyapatite graft for the treatment of 3-Wall Intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2017;88:1288–96. 10.1902/jop.2012.110722 [DOI] [PubMed] [Google Scholar]
- 23.Liu K, Huang Z, Chen Z. Treating periodontal intrabony defects using guided tissue regeneration and Bio-Oss® with platelet-rich fibrin: study protocol for a self-controlled trial. Clin Trials Orthop Disord 2020;5:9–13. [Google Scholar]
- 24.Liu K, Huang Z, Chen Z, et al. Treatment of periodontal intrabony defects using bovine porous bone mineral and guided tissue regeneration with/without platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol 2021;92:1546–53. 10.1002/JPER.20-0860 [DOI] [PubMed] [Google Scholar]
- 25.Varghese MP, Manuel S, Kumar L K S. Potential for osseous regeneration of platelet-rich Fibrin-A comparative study in mandibular third molar impaction sockets. J Oral Maxillofac Surg 2017;75:1322–9. 10.1016/j.joms.2017.01.035 [DOI] [PubMed] [Google Scholar]
- 26.Bilginaylar K, Uyanik LO. Evaluation of the effects of platelet-rich fibrin and piezosurgery on outcomes after removal of ımpacted mandibular third molars. Br J Oral Maxillofac Surg 2016;54:629–33. 10.1016/j.bjoms.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 27.Uppada UK, Kalakonda B, Koppolu P, et al. Combination of hydroxyapatite, platelet rich fibrin and amnion membrane as a novel therapeutic option in regenerative periapical endodontic surgery: case series. Int J Surg Case Rep 2017;37:139–44. 10.1016/j.ijscr.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiremath H, Motiwala T, Jain P, et al. Use of second-generation platelet concentrate (platelet-rich fibrin) and hydroxyapatite in the management of large periapical inflammatory lesion: a computed tomography scan analysis. Indian J Dent Res 2014;25:517–20. 10.4103/0970-9290.142556 [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod 2006;32:601–23. 10.1016/j.joen.2005.12.010 [DOI] [PubMed] [Google Scholar]
- 30.Nakkeeran KP, Saravanan K, Babu P, et al. Evaluation of bone regeneration in periapical osseous defects with and without platelet rich plasma, combined calcium sulfate and autologous bone graft - A comparative study. J Stomatol Oral Maxillofac Surg 2019;120:196–202. 10.1016/j.jormas.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 31.Kim NR, Lim B-S, Park HC, et al. Effects of N-acetylcysteine on TEGDMA- and HEMA-induced suppression of osteogenic differentiation of human osteosarcoma MG63 cells. J Biomed Mater Res B Appl Biomater 2011;98:300–7. 10.1002/jbm.b.31852 [DOI] [PubMed] [Google Scholar]
- 32.Jayalakshmi KB, Agarwal S, Singh MP, et al. Platelet-Rich fibrin with β-Tricalcium Phosphate-A Noval approach for bone augmentation in chronic periapical lesion: a case report. Case Rep Dent 2012;2012:902858. 10.1155/2012/902858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortellini S, Castro AB, Temmerman A, et al. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: a proof-of-concept study. J Clin Periodontol 2018;45:624–34. 10.1111/jcpe.12877 [DOI] [PubMed] [Google Scholar]
- 34.Lei L, Yu Y, Ke T, et al. The application of three-dimensional printing model and platelet-rich fibrin technology in guided tissue regeneration surgery for severe bone defects. J Oral Implantol 2019;45:35–43. 10.1563/aaid-joi-D-17-00231 [DOI] [PubMed] [Google Scholar]
- 35.Sezgin Y, Uraz A, Taner IL, et al. Effects of platelet-rich fibrin on healing of intra-bony defects treated with anorganic bovine bone mineral. Braz Oral Res 2017;31:e15. 10.1590/1807-3107bor-2017.vol31.0015 [DOI] [PubMed] [Google Scholar]
- 36.Fickl S, Kebschull M, Schupbach P, et al. Bone loss after full-thickness and partial-thickness flap elevation. J Clin Periodontol 2011;38:157–62. 10.1111/j.1600-051X.2010.01658.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.