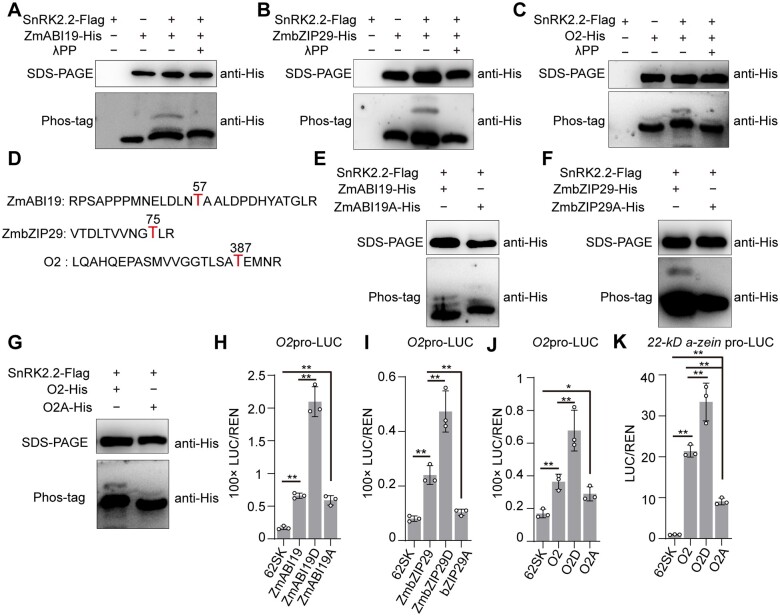
Figure 10.
Phosphorylation of ZmABI19, ZmbZIP29, and O2 by SnRK2.2 increases transactivation capacity. A, In vitro assay showing phosphorylation of ZmABI19 by SnRK2.2. The phosphorylation of ZmABI19 was assayed in the absence or presence of SnRK2.2-Flag. Lambda protein phosphatase λPP was added to dephosphorylate ZmABI19. B, In vitro assay showing phosphorylation of ZmbZIP29 by SnRK2.2. C, In vitro assay showing phosphorylation of O2 by SnRK2.2. D, The phosphorylated peptides and residues (red) in ZmABI19, ZmbZIP29, and O2. E–G, Substitution of the phosphorylatable threonine with alanine in ZmABI19 (E), ZmbZIP29 (F), and O2 (G) resulted in inhibited phosphorylation. H–K, Altered transactivation capacities of phosphorylation-mimetic (T substituted with D) and dephosphorylated (T substituted with A) forms of ZmABI19 (H), ZmbZIP29 (I), and O2 (J and K). 10-μM ABA was used in these assays. The relative ratio of LUC/REN was tested in Arabidopsis leaf mesophyll protoplasts via co-transforming the reporter plasmids with the effector construct. Error bars represent SD of three biological replicates. Statistical significance was determined with Student’s t test. *P < 0.05; **P < 0.01.