Figure 4.
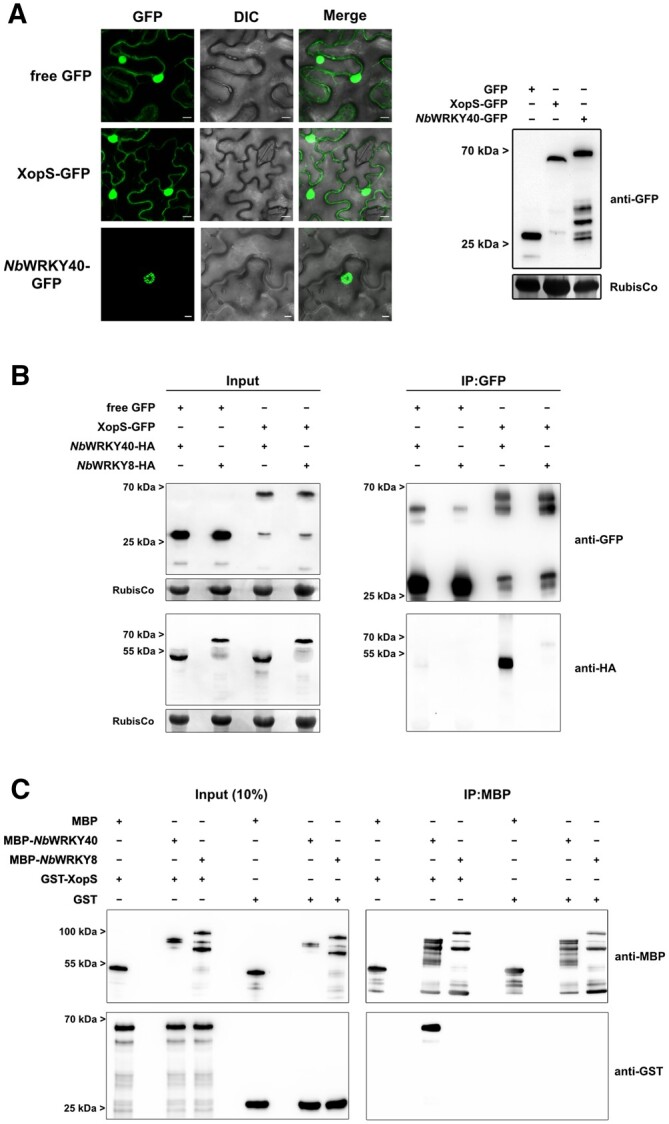
WRKY40 interacts with XopS in planta and in vitro. A, Subcellular localization of XopS-GFP and NbWRKY40-GFP. GFP fusion proteins or free GFP under control of the CaMV35S promoter were expressed transiently in leaves of N. benthamiana using Agrobacterium-infiltration. The localization of transiently expressed proteins (left) was determined with confocal laser scanning microscopy 24 hpi. Scale bars represent 20 µm. DIC, Differential Interference Contrast. A representative image from 10 randomly chosen regions of interest of infiltrated leaves is shown. The experiment was carried out at least 3 times with similar results. Protein expression was verified by immunoblotting using an anti-GFP antibody (right). Amido black staining of RubisCo served as a loading control. B, Co-IP of either free GFP or XopS-GFP with NbWRKY40-HA or NbWRKY8-HA. Proteins were transiently co-expressed in leaves of N. benthamiana using Agrobacterium-infiltration. After 24 h, total protein extracts (Input) were subjected to IP (IP:GFP) with GFP-Trap beads, followed by immunoblotting using either anti-GFP or anti-HA antibodies. Amido black staining of RubisCo served as a loading control for input samples. The experiment was carried out at least 2 times with similar results. C, In vitro pull-down assay showing physical interaction of XopS with NbWRKY40. MBP, MBP-NbWRKY40, MBP-NbWRKY8, GST, and GST-XopS were expressed in E. coli. Pull-down was performed by affinity purification of MBP-tagged proteins using amylose resin. MBP alone and GST alone were used as negative controls. Pull-down experiments with MBP-NbWRKY8 were additionally performed to confirm specificity of interaction between NbWRKY40 and XopS. Indicated recombinant proteins were detected before (Input 10%) and after affinity purification (IP:MBP) by immunoblotting using anti-MBP or anti-GST antibodies. The experiment was carried out twice with similar results.
