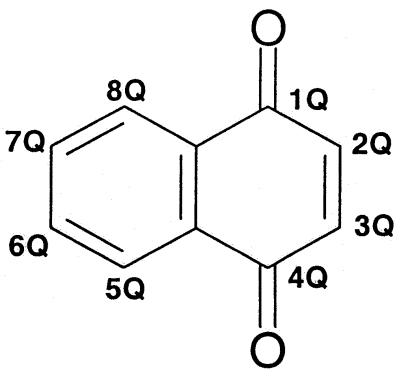
TABLE 1.
Antipneumocystis activities of 1,4-naphthoquinone derivatives
| Compounda | Substituent (at position specified on above parent structureb)
|
% Inhibition at the following concn [μg/ml]):
|
||||
|---|---|---|---|---|---|---|
| 2Q | 3Q | 7Q | 0.1 | 1 | 10 | |
| ATQ | −OH | 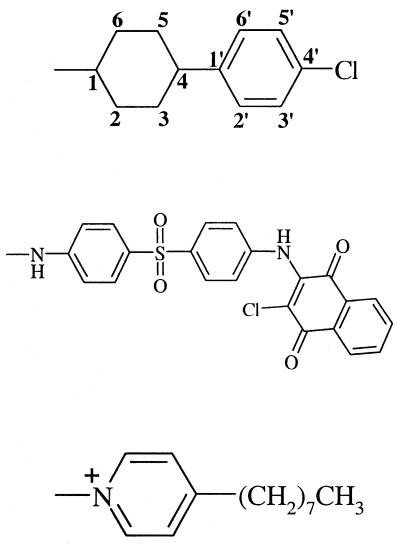 |
−H | 27 | 92 | −c |
| 1 (39355) | −Cl | −H | 42 | 88 | − | |
| 2 (142574) | −O− | −H | 42 | 79 | − | |
| 3 (278) | −OH | 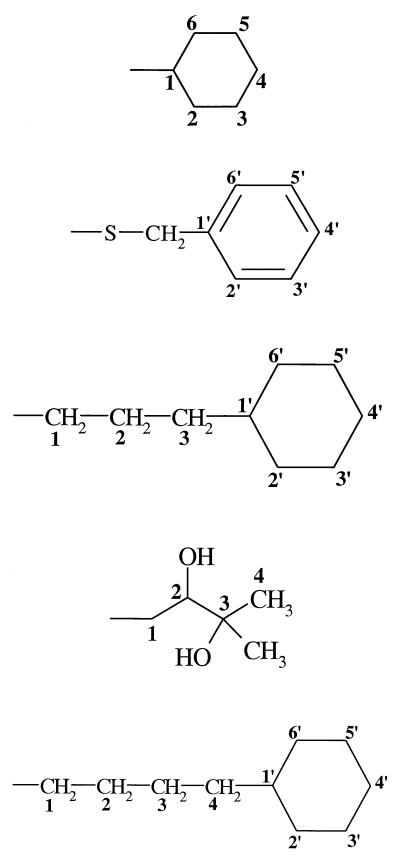 |
−H | 28 | 51 | − |
| 4 (66457) | −CH3 | −H | 27 | 43 | − | |
| 5 (109542) | −OH | −OCH3 | 25 | 39 | − | |
| 6 (100407) | −OH | −H | 11 | 39 | − | |
| 7 (31435) | −OH | −H | − | 28 | − | |
| 8 (359831) | −Cl | 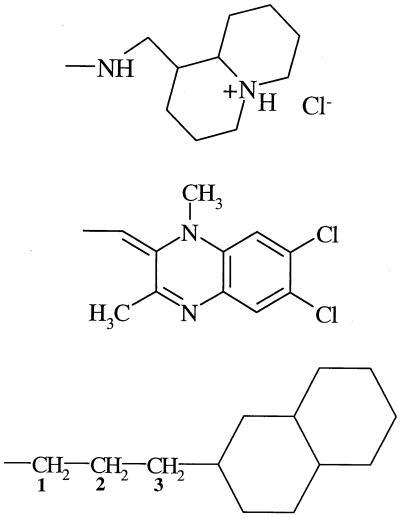 |
−H | − | 25 | − |
| 9 (295483) | −Cl | −H | − | 24 | − | |
| 10 (2035) | −OH | −H | − | 18 | − | |
| 11 (26695) | −OH | 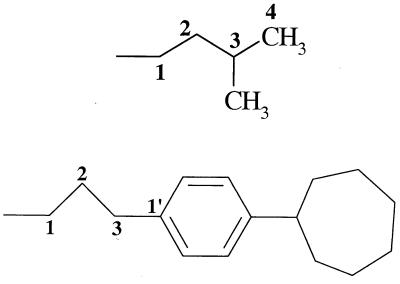 |
−H | − | 14 | − |
| 12 (113452) | −OH | −H | − | 13 | − | |
| 13 (92195) | −Cl | −H | − | 10 | − | |
| 14 (126771) | −OH | −H | − | 3 | − | |
| 15 (26654) | −OH | −H | − | − | 14 | |
| 16 (31855) | −OH | −H | − | − | 12 | |
| 17 (114930) | −OH | 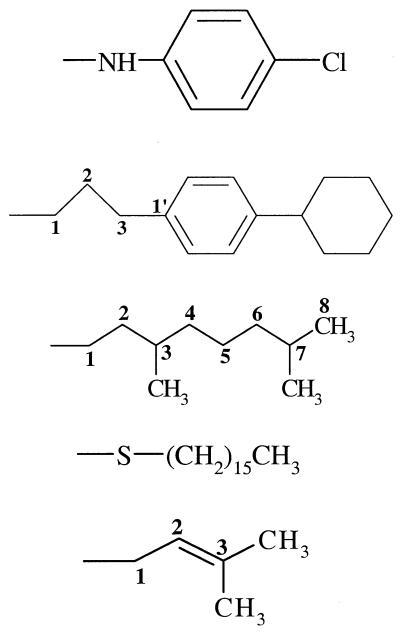 |
−H | − | − | 6 |
| 18 (113455) | −OH | −H | − | −5 | − | |
| 19 (138685) | −OH | −H | − | −15 | − | |
| 20 (247507) | −NH2 | −H | − | − | −23 | |
| 21 (629756) | −OH | −H | − | − | −23 | |
Numbers in parentheses are the National Cancer Institute NSC entry numbers.
A Q is suffixed to each locant on the 1,4-naphthoquinone nucleus to distinguish it from the locant bearing the same numeral but identifying a position in the substituent.
−, not tested.