Abstract
Objective
Although cognitive impairment has received more attention in recent years as a result of spinal cord injury (SCI), the pathogenic process that causes it is still unknown. The neuroprotective effects of Netrin as a family of laminin-related secreted proteins were discovered. The purpose of this study was to determine the changes of serum Netrin-1 after SCI and its relationship with cognitive impairment.
Methods
96 SCI patients and 60 controls were included in our study. We collected baseline data from all participants, measured their serum Netrin-1 levels, and followed up their cognitive levels 3 months later.
Results
The clinical baseline values between the control and SCI groups were not significantly different (p > 0.05). However, the serum Netrin-1 level in the SCI group was significantly lower than that in the control group (528.4 ± 88.3 pg/ml vs. 673.5 ± 97.2 pg/ml, p < 0.05). According to the quartile level of serum Netrin-1 level in the SCI group, we found that with the increase of serum Netrin-1 level, the MoCA score also increased significantly (p < 0.001), indicating that the serum Netrin-1 level was positively correlated with the MoCA score after SCI. After controlling for baseline data, multiple regression analysis revealed that Netrin-1 remained an independent risk factor for cognitive impairment after SCI (=0.274, p = 0.036).
Conclusions
Netrin-1 may be a neuroprotective factor for cognitive impairment, which may serve as a serum marker to predict cognitive impairment after SCI.
1. Introduction
Cognitive impairment is a widespread complication following spinal cord injury (SCI), affecting approximately 10-60% of patients [1, 2]. Before the 1940s, only a small percentage of patients could survive for a few weeks after injury, but advances in technology have greatly improved the survival rate of SCI [3, 4]. According to statistics, up to 90% of people can survive for 1 year after injury [5]. The potential cognitive impairment of survivors becomes an important part of SCI rehabilitation. Cognitive decline following SCI is diverse, affecting areas including memory, language, abstract reasoning, attention, emotion, concentration, and problem solving [6–8]. Therefore, in order to improve the quality of life after SCI injury, it is particularly important and urgent to find biomarkers for predicting cognitive impairment and formulate targeted treatment and rehabilitation strategies.
Professor Tessier-Lavigne discovered Netrin-1, a membrane protein-like protein, in 1994, and it was initially recognized as a potent chemotactic molecule involved in axon guidance and cell migration during embryonic development [9, 10]. Netrin-1 consists of 604 amino acids and is encoded by the NTN1 gene, which is highly evolutionarily conserved [11–13]. Netrin-1 is mostly expressed in neurons and oligodendrocytes in animals, and it has lately been linked to angiogenesis, inflammation, tissue remodeling, and cancer [14]. Netrin-1 acts as both an attractor and a repulsor, and its function depends on its receptors [15, 16]. Although Netrin-1 was discovered nearly 30 years, its function is still poorly understood.
Although data on Netrin-1's significance in neurological illnesses and SCI have begun to appear in recent years, its association with cognitive impairment remains uncertain [17]. The goal of this study was to look at how serum Netrin-1 changed in the acute phase after SCI and how that related to cognitive impairment. The study of Netrin-1 as a potential serum marker of cognitive impairment after SCI will be helpful for the treatment and rehabilitation of cognitive impairment after SCI.
2. Methods
2.1. Study Population
Spinal cord injury (SCI) patients presenting to Neck-Shoulder and Lumbocrural Pain Hospital were enrolled in this study. Inclusion criteria are as follows: age > 18 years old; patients with spinal fractures confirmed by CT examination; seek medical treatment within 24 hours after onset; and volunteer to participate in this study. Exclusion criteria were previous neurological trauma or surgical history, severe organic diseases such as heart, liver, and kidney, unstable vital signs, and death within 3 months of injury. In addition, 60 healthy controls were recruited to voluntarily join the study. The hospital's ethics committee approved our research. Informed consent was given by all patients or family members.
2.2. Baseline Data Collection
All participants received a questionnaire to collect baseline data at enrollment. These data included age, gender, smoking, drinking, and whether there was a history of hypertension, diabetes, coronary heart disease, and hyperlipidemia. The collection of clinical baseline data was double-checked to ensure accuracy.
2.3. Serum Netrin-1 Level Determination
All participants received fasting venous blood collection within 24 days of enrollment. After whole blood was collected, coagulation was performed for 20 minutes at room temperature. Blood clots were removed after whole blood was centrifuged at 2000 × g for 10 minutes in a refrigerated centrifuge. The resulting supernatant was designated serum. The determination of Netrin-1 adopts enzyme-linked immunosorbent assay (ELISA) method, and the antibody adopts commercial product. The specific ELISA steps refer to the instructions and previous reports [18, 19].
2.4. MoCA Scale Evaluation
MoCA is a simple tool for screening for cognitive impairment and is intended for use by health professionals only. This 10-minute, 30-point assessment tool assesses different cognitive domains, including attention, concentration, conceptual thinking, executive function, visual construction skills, memory, language, calculation, and orientation. MoCA has so far been available in 46 languages [20]. In this study, we used the MoCA scale to evaluate cognitive function 3 months after SCI. All scale reviewers were uniformly trained to reduce bias.
2.5. Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables were expressed as percentages. A normality test was performed on these data. The t-test was used to assess group differences for continuous variables when the normal distribution was fitted. Categorical variables were compared using the chi-square test. A trend test was used to evaluate the association of Netrin-1 with MoCA. Statistical significance was established when p < 0.05. Statistical analysis was performed using the SPSS 22.0 program package.
3. Results
3.1. Clinical Baseline Data
The control and SCI groups each had 60 and 96 participants, respectively. Between the two groups, there were no significant differences in age, gender, smoking and drinking habits, or past medical history (hypertension, diabetes, coronary heart disease, and hyperlipidemia) (p > 0.05). Table 1 contains the detailed statistics.
Table 1.
Baseline data from the study population.
| Controls (n = 60) | SCI (n = 96) | p value | |
|---|---|---|---|
| Age, years | 58.2 ± 6.4 | 58.6 ± 6.9 | 0.718 |
| Gender, male/female | 49/11 | 81/15 | 0.659 |
| Drinking, n (%) | 41 | 70 | 0.539 |
| Smoking, n (%) | 37 | 62 | 0.713 |
| Hypertension, n (%) | 19 | 31 | 0.935 |
| Diabetes, n (%) | 13 | 19 | 0.778 |
| Coronary heart disease, n (%) | 10 | 18 | 0.741 |
| Hyperlipidemia, n (%) | 26 | 38 | 0.643 |
| Netrin-1 (pg/ml) | 673.5 ± 97.2 | 528.4 ± 88.3 | <0.001 |
| MoCA (points) | 27.3 ± 1.1 | 23.4 ± 1.8 | <0.001 |
However, the MoCA scores of the SCI group and the control group were 23.4 ± 1.8 and 27.3 ± 1.1 points, respectively, and the MoCA score of the SCI group was significantly lower than that of the control group (p < 0.001); however, the serum Netrin-1 levels in the SCI group and the control group were 528.4 ± 88.3 pg/ml and 673.5 ± 97.2 pg/ml, respectively, and the serum Netrin-1 level in the SCI (Table 1 and Figure 1) indicates the differences in MoCA scores and serum Netrin-1 levels between the two groups.
Figure 1.
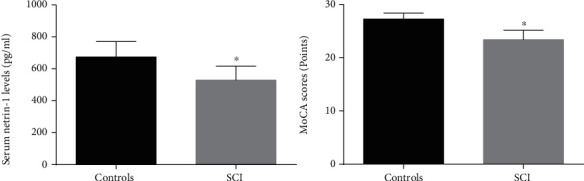
Comparison of serum Netrin-1 levels with MoCA score. ∗p < 0.05 compared to controls.
3.2. Correlation between MoCA and Serum Netrin-1 Level
According to the quartiles of serum Netrin-1 levels in SCI patients, we divided SCI patients into 4 groups, from low to high: Q1, Q2, Q3, and Q4 groups. We also analyzed the MoCA scores of different groups. The p for trend test was used to verify the correlation between serum Netrin-1 level trends and MoCA scores. The results showed that serum Netrin-1 level was significantly correlated with MoCA score (p < 0.001). The correlation analysis between serum Netrin-1 level and MoCA is shown in Table 2.
Table 2.
Relationship between serum Netrin-1 levels and MoCA scores.
| Variable | Q1 | Q2 | Q3 | Q4 | p values |
|---|---|---|---|---|---|
| MoCA scores | 22.5 ± 1.7 | 23.1 ± 2.0 | 23.8 ± 1.9 | 24.2 ± 1.6 | <0.001 |
3.3. Multiple Regression Analysis
We incorporated clinical baseline data into regression equations to analyze potential etiologies affecting cognitive function after SCI. The results (Table 3) showed that age, gender, smoking, drinking, diabetes, hypertension, coronary heart disease, and hyperlipidemia were not the key factors affecting cognitive impairment after SCI. Serum Netrin-1 level could still independently predict cognitive impairment after SCI after adjusting for the above interference factors (β = 0.274, p = 0.036).
Table 3.
Multiple regression analysis for predicting cognitive impairment in SCI patients.
| Regression coefficient | 95% confidence interval | p value | |
|---|---|---|---|
| Age | 0.132 | 0.107-1.016 | 0.163 |
| Gender | 0.205 | 0.148-1.098 | 0.312 |
| Drinking | 0.227 | 0.183-1.034 | 0.405 |
| Smoking | 0.218 | 0.109-1.105 | 0.199 |
| Hypertension | 0.186 | 0.124-1.073 | 0.227 |
| Diabetes | 0.260 | 0.185-1.121 | 0.174 |
| Coronary heart disease | 0.191 | 0.086-1.062 | 0.338 |
| Hyperlipidemia | 0.243 | 0.162-1.240 | 0.081 |
| Netrin-1 | 0.274 | 0.113-0.853 | 0.036 |
4. Discussion
This study was a single-center clinical trial on the hot topic of cognitive impairment following spinal cord injury. The goal of this study was to see if there was a link between serum Netrin-1 and cognitive impairment following a spinal cord injury. The findings revealed that in the acute phase of SCI patients, serum Netrin-1 levels were much lower, and this decline was significantly linked with the MoCA score. The serum Netrin-1 level could predict cognitive damage after SCI independently, according to regression analysis. Our research is the first to show a link between Netrin-1 and cognitive impairment following a spinal cord injury.
For decades, developmental neurobiologists have been searching for chemical molecules with axon guidance mechanisms. In 1994, the first biochemical purification of axonal growth-promoting factors was identified in chicken embryos, named Netrins (Netrin-1 and Netrin-2), a laminin-related molecule involved in axon guidance and cell migration [17, 21, 22]. Netrin-1, the most studied member of the family, is highly conserved during development and is expressed in neuroepithelial cells of the floor plate and ventral region of the spinal cord. In the nervous system, it is mainly expressed in neurons and oligodendrocytes; outside the nervous system, Netrin-1 is expressed in the limb primordia, breast, pancreas, dorsal aorta, and ovary [17, 23, 24]. Netrin-1 has dual functions depending on the receptor. Binding of Netrin-1 alone to DCC results in axonal attraction that recruits intracellular signaling complexes to activate and trigger cytoskeletal rearrangements [25, 26]. However, when un5a D coexists with DCC, Netrin-1 enables the DCC P1 motif to interact with the DB domain of Unc5A D, initiating the Netrin-1 exclusion signaling pathway [25, 27, 28]. Although Netrin-1 has been discovered for many years, its specific regulatory mechanism in vivo has not been fully elucidated.
The role of Netrin-1 in neurological diseases has been widely reported. Evidence suggests that Netrin-1 may be a promising drug candidate for reducing stroke severity and improving prognosis, and its neuroprotective mechanisms may be achieved through regulation of angiogenesis, autophagy, apoptosis, and neuroinflammation [29]. Glioblastoma is one of the most common tumors in neurosurgery, and evidence in recent years suggests that Netrin-1, as an atypical angiogenic ligand, may be involved in the promotion of neovascularization in glioblastoma [30]. Evidence that Netrin-1 is involved in the pathogenesis of Alzheimer's disease has been found in cells, animals, and clinical experiments, indicating that Netrin-1 may be involved in the regulation of neuroplasticity [31–33]. Studies on the regulation of Netrin-1 receptors in Parkinson's disease have also been reported [33–35]. Another study pointed out that serum Netrin-1 concentration decreased after brain injury and could be used as a biomarker of traumatic brain injury [36]. Further animal experiments showed that the mechanism of Netrin-1 protecting traumatic brain injury may be related to its protection of the integrity of the blood-brain barrier [37]. Netrin-1 and its receptor are involved in the maintenance of neural function as an important neural regulatory axis.
The role of Netrin-1 in SCI is beginning to emerge. In order to explore the potential role of Netrin-1 after SCI, Canadian scholars detected the expression of Netrin-1 and receptors in rats after spinal cord amputation. The results showed that Netrin-1 was significantly decreased at the injury site and sustained a low decrease for 7 months and was mainly expressed in neurons and oligodendrocytes immediately adjacent to the lesion [38]. Chinese researchers explored the therapeutic effect of Netrin-1 in a rat model of spinal cord injury in a study. The results show that the motor function of injured rats can be significantly improved, and the mechanism may be achieved by activating the AMPK/mTOR signaling pathway to regulate autophagy [39, 40]. The expression of Netrin-1 protein in the spinal cord of rats with experimental autoimmune encephalomyelitis (EAE) was studied by a Korean research team. Netrin-1 levels in the spinal cord of rats at the height of EAE have been found to be greatly elevated, whereas normal human Netrin-1 is mostly expressed in spinal neurons, oligodendrocytes, astrocytes, and vascular endothelial cells [41]. The regulatory role of Netrin-1 in EAE injury in rats has been discovered, but its research in humans has not been reported yet.
Our study is the first to report the involvement of Netrin-1 in cognitive impairment after SCI. Despite the obvious strengths of our study, the limitations of our study still need to be stated. First, we are not a multicenter large-sample study; second, we have no mechanistic studies involving animal or cell experiments; and finally, our study lacks detection data for Netrin receptors.
5. Conclusions
In the acute phase of SCI, serum Netrin-1 levels were much lower, and this was linked to a reduction in cognitive performance. The level of serum Netrin-1 may be a serum marker for predicting cognitive impairment after SCI, and a deeper understanding of its mechanism could modify the prevention and treatment strategy for cognitive impairment after SCI.
Acknowledgments
This research was funded by the Academic Promotion Project of Shandong First Medical University (2019QL003), the Shandong Provincial Central Government Guides Local Science and Technology Development Fund Projects (YDZX20203700002055) and the Qilu Medical School Traditional Chinese Medicine Academic School Inheritance Project.
Contributor Information
Shengnan Cao, Email: feiyan.70172@qq.com.
Bin Shi, Email: sdyky-shibin@163.com.
Data Availability
The study data presented may be made available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors report no conflict of interest.
References
- 1.Nightingale T. E., Zheng M. M. Z., Sachdeva R., Phillips A. A., Krassioukov A. V. Diverse cognitive impairment after spinal cord injury is associated with orthostatic hypotension symptom burden. Physiology & Behavior . 2020;213, article 112742 doi: 10.1016/j.physbeh.2019.112742. [DOI] [PubMed] [Google Scholar]
- 2.Liu F. J., Xu H. H., Yin Y., et al. Decreased adiponectin levels are a risk factor for cognitive decline in spinal cord injury. Disease Markers . 2022;2022:6. doi: 10.1155/2022/5389162.5389162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North N. The psychological effects of spinal cord injury: a review. Spinal Cord . 1999;37(10):671–679. doi: 10.1038/sj.sc.3100913. [DOI] [PubMed] [Google Scholar]
- 4.Cao S., Shi H., Sun G., et al. Serum IL-37 level is associated with rheumatoid arthritis and disease activity: a meta-analysis. BioMed Research International . 2021;2021:7. doi: 10.1155/2021/6653439.6653439 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Frankel H., Coll J., Charlifue S., et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord . 1998;36(4):266–274. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- 6.Sachdeva R., Gao F., Chan C. C., Krassioukov A. V. Cognitive function after spinal cord injury: a systematic review. Neurology . 2018;91(13):611–621. doi: 10.1212/WNL.0000000000006244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig A., Guest R., Tran Y., Middleton J. Cognitive impairment and mood states after spinal cord injury. Journal of Neurotrauma . 2017;34(6):1156–1163. doi: 10.1089/neu.2016.4632. [DOI] [PubMed] [Google Scholar]
- 8.Davidoff G. N., Roth E. J., Richards J. S. Cognitive deficits in spinal cord injury: epidemiology and outcome. Archives of Physical Medicine and Rehabilitation . 1992;73(3):275–284. [PubMed] [Google Scholar]
- 9.Bradford D., Cole S. J., Cooper H. M. Netrin-1: diversity in development. The International Journal of Biochemistry & Cell Biology . 2009;41(3):487–493. doi: 10.1016/j.biocel.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Serafini T., Colamarino S. A., Leonardo E. D., et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell . 1996;87(6):1001–1014. doi: 10.1016/S0092-8674(00)81795-X. [DOI] [PubMed] [Google Scholar]
- 11.Marino S. M., Gladyshev V. N. Crystal structure of N-glycosylated human glypican-1 core protein. The Journal of Biological Chemistry . 2012;287(17):14040–14051. doi: 10.1074/jbc.M111.322487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinstein J., Ramkhelawon B. Netrins & Semaphorins: Novel regulators of the immune response. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease . 2017;1863(12):3183–3189. doi: 10.1016/j.bbadis.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorla M., Bashaw G. J. Molecular mechanisms regulating axon responsiveness at the midline. Developmental Biology . 2020;466(1-2):12–21. doi: 10.1016/j.ydbio.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manitt C., Colicos M. A., Thompson K. M., Rousselle E., Peterson A. C., Kennedy T. E. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. Journal of Neuroscience . 2001;21(11):3911–3922. doi: 10.1523/JNEUROSCI.21-11-03911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Y., Shepherd I., Li J., Renzi M. J., Chang S., Raper J. A. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron . 1995;14(6):1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- 16.Vishwanathan A. Micro-technologies to constrain neuronal networks . University of Pittsburgh; 2011. [Google Scholar]
- 17.Dun X.-P., Parkinson D. B. Role of Netrin-1 signaling in nerve regeneration. International Journal of Molecular Sciences . 2017;18(3):p. 491. doi: 10.3390/ijms18030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y., Wang K., Wang Q., Ma Y., Liu X. The antioxidant enzyme PON1: a potential prognostic predictor of acute ischemic stroke. Oxidative Medicine and Cellular Longevity . 2021;2021:8. doi: 10.1155/2021/6677111.6677111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun S., Sun S., Meng Y., Shi B., Chen Y. Elevated serum neuropeptide FF levels are associated with cognitive decline in patients with spinal cord injury. Disease Markers . 2021;2021:7. doi: 10.1155/2021/4549049.4549049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Q., Wang K., Ma Y., Li S., Xu Y. Serum galectin-3 as a potential predictive biomarker is associated with poststroke cognitive impairment. Oxidative Medicine and Cellular Longevity . 2021;2021:7. doi: 10.1155/2021/5827812.5827812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nature Reviews Cancer . 2004;4(12):978–987. doi: 10.1038/nrc1504. [DOI] [PubMed] [Google Scholar]
- 22.Boyer N. P., Gupton S. L. Revisiting netrin-1: one who guides (axons) Frontiers in Cellular Neuroscience . 2018;12 doi: 10.3389/fncel.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brignani S., Raj D. D., Schmidt E. R., et al. Remotely produced and axon-derived netrin-1 instructs GABAergic neuron migration and dopaminergic substantia nigra development. Neuron . 2020;107, article e689:684–702. doi: 10.1016/j.neuron.2020.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Kang D. S., Yang Y. R., Lee C., et al. Netrin-1/DCC-mediated PLC γ1 activation is required for axon guidance and brain structure development. EMBO Reports . 2018;19(11, article e46250) doi: 10.15252/embr.201846250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H.-J., Qu N., Hui L., et al. Further confirmation of netrin 1 receptor (DCC) as a depression risk gene via integrations of multi-omics data. Translational Psychiatry . 2020;10(1):1–15. doi: 10.1038/s41398-020-0777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torres-Berrío A., Hernandez G., Nestler E. J., Flores C. The netrin-1/DCC guidance cue pathway as a molecular target in depression: translational evidence. Biological Psychiatry . 2020;88(8):611–624. doi: 10.1016/j.biopsych.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finci L., Zhang Y., Meijers R., Wang J.-H. Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Progress in Biophysics and Molecular Biology . 2015;118(3):153–160. doi: 10.1016/j.pbiomolbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barallobre M. J., Pascual M., Del Río J. A., Soriano E. The netrin family of guidance factors: emphasis on netrin-1 signalling. Brain Research Reviews . 2005;49(1):22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y., Liao S., Yu J. Netrin-1 in post-stroke neuroprotection: beyond axon guidance cue. Current Neuropharmacology . 2022;20 doi: 10.2174/1570159X20666220302150723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vásquez X., Sánchez-Gómez P., Palma V. Netrin-1 in glioblastoma neovascularization: the new partner in crime? International Journal of Molecular Sciences . 2021;22(15):p. 8248. doi: 10.3390/ijms22158248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamani E., Parviz M., Roghani M., Hosseini M., Mohseni-Moghaddam P., Nikbakhtzadeh M. Netrin-1 protects the SH-SY5Y cells against amyloid beta neurotoxicity through NF-κB/Nrf2 dependent mechanism. Molecular Biology Reports . 2020;47(12):9271–9277. doi: 10.1007/s11033-020-05996-1. [DOI] [PubMed] [Google Scholar]
- 32.Sun L., Ju T., Wang T., et al. Decreased netrin-1 and correlated Th17/Tregs balance disorder in Aβ1–42 induced Alzheimer's disease model rats. Frontiers in Aging Neuroscience . 2019;11:p. 124. doi: 10.3389/fnagi.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ju T., Sun L., Fan Y., et al. Decreased netrin-1 in mild cognitive impairment and Alzheimer's disease patients. Frontiers in Aging Neuroscience . 2021;13, article 762649 doi: 10.3389/fnagi.2021.762649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lo P. S., Rymar V. V., Kennedy T. E., Sadikot A. F. The netrin-1 receptor DCC promotes the survival of a subpopulation of midbrain dopaminergic neurons: relevance for ageing and Parkinson's disease. Journal of Neurochemistry . 2022 doi: 10.1111/jnc.15579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G., Ahn E. H., Kang S. S., et al. UNC5C Receptor Proteolytic Cleavage by Active AEP Promotes Dopaminergic Neuronal Degeneration in Parkinson's Disease. Advanced Science . 2022;9(7, article e2103396) doi: 10.1002/advs.202103396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie Y., Guo Z., Chen F., Xiao C., Xu J., Bo D. Serum netrin-1 as a potential biomarker for functional outcome of traumatic brain injury. Clinica Chimica Acta . 2021;518:22–27. doi: 10.1016/j.cca.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Wen J., Qian S., Yang Q., Deng L., Mo Y., Yu Y. Overexpression of netrin-1 increases the expression of tight junction-associated proteins, claudin-5, occludin, and ZO-1, following traumatic brain injury in rats. Experimental and Therapeutic Medicine . 2014;8(3):881–886. doi: 10.3892/etm.2014.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manitt C., Wang D., Kennedy T. E., Howland D. R. Positioned to inhibit: netrin-1 and netrin receptor expression after spinal cord injury. Journal of Neuroscience Research . 2006;84(8):1808–1820. doi: 10.1002/jnr.21070. [DOI] [PubMed] [Google Scholar]
- 39.Bai L., Mei X., Shen Z., et al. Netrin-1 improves functional recovery through autophagy regulation by activating the AMPK/mTOR signaling pathway in rats with spinal cord injury. Scientific Reports . 2017;7(1):1–10. doi: 10.1038/srep42288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bai L., Mei X., Wang Y., et al. The role of netrin-1 in improving functional recovery through autophagy stimulation following spinal cord injury in rats. Frontiers in Cellular Neuroscience . 2017;11:p. 350. doi: 10.3389/fncel.2017.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon C., Ahn M., Jeong C., Kim H., Shin T. Immunohistochemical study of netrin-1 in the spinal cord with rat experimental autoimmune encephalomyelitis. Immunological Investigations . 2011;40(2):160–171. doi: 10.3109/08820139.2010.525570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study data presented may be made available from the corresponding author upon reasonable request.


