Abstract
Loss of midbrain dopamine neurons causes the cardinal symptoms of Parkinson’s disease. However, not all dopamine neurons are equally vulnerable and a better understanding of the cell-type specific properties relating to selective dopamine neuron degeneration is needed. Most midbrain dopamine neurons express the vesicular glutamate transporter VGLUT2 during development and a subset continue to express low levels of VGLUT2 in adulthood, enabling the co-release of glutamate. Moreover, VGLUT2 expression in dopamine neurons can be neuroprotective since its genetic disruption was shown to sensitize dopamine neurons to neurotoxins. Here, we show that in response to toxic insult, and in two distinct models of alpha-synuclein stress, VGLUT2 dopamine neurons were resilient to degeneration. Dopamine neurons expressing VGLUT2 were enriched whether or not insult induced dopamine neuron loss, suggesting that while VGLUT2 dopamine neurons are more resilient, VGLUT2 expression can also be transcriptionally upregulated by injury. Finally, we observed that VGLUT2 expression was enhanced in surviving dopamine neurons from post-mortem Parkinson’s disease individuals. These data indicate that emergence of a glutamatergic identity in dopamine neurons may be part of a neuroprotective response in Parkinson’s disease.
Keywords: dopamine, vesicular glutamate transporter 2, selective vulnerability, Parkinson's disease, alpha-synuclein
Steinkellner et al. report that dopaminergic neurons capable of co-releasing glutamate are more resilient to degeneration in animal models and in patients with Parkinson's disease, suggesting that a glutamatergic identity may be neuroprotective.
Introduction
As a result of population ageing, the global burden of Parkinson’s disease has more than doubled from 1990 to 2016, and it is expected that the number of patients suffering from Parkinson’s disease will increase by 40–50% over the next 20–30 years.1 The cause of Parkinson’s disease is unknown and there is no treatment to prevent, stop or reverse its course. Current pharmacotherapy provides only transient symptomatic relief and can itself aggravate the condition through drug-induced dyskinesia.2 Therefore, discovery of novel targets for developing new therapeutic strategies is needed.
Many neuronal populations can be affected in Parkinson’s disease; however, the cardinal motor symptoms are a consequence of progressive dopamine (DA) neuron loss in the midbrain substantia nigra pars compacta (SNc). The precise mechanisms underlying DA neuron vulnerability remain unclear, but include mitochondrial dysfunction, oxidative stress and aggregation of α-synuclein.3 Importantly, midbrain DA neurons are neurochemically diverse4 and are not all equally vulnerable to degeneration. For example, DA neurons in the ventral tier of SNc tend to be most vulnerable, while those medial to the SNc in the ventral tegmental area (VTA) are relatively spared.5-7
There is now definitive evidence in adult flies, mice, rats, monkeys and humans that subsets of midbrain DA neurons express the vesicular glutamate transporter VGLUT2,8,9 and that VGLUT2 is necessary and sufficient for cotransmission of glutamate.10 While enabling vesicular glutamate release, VGLUT2 can also facilitate packaging of DA into the same vesicles through effects on the proton electrochemical gradient,11,12 and this process may ‘tune’ DA quantal content in response to increased demand.13
VGLUT2 is more abundant in medial VTA DA neurons that have reduced propensity to degenerate in Parkinson’s disease and Parkinson’s disease animal models, though VGLUT2+ DA neurons can also be found in the SNc.4,14,15 Moreover, most SNc and VTA DA neurons transiently express VGLUT2 during development, suggesting that most mature midbrain DA neurons repress its expression.15,16 Further, DA neuron-selective toxicants spare VGLUT2+ DA neurons—while genetic disruption of VGLUT2 from DA neurons increases their vulnerability to these neurotoxins suggesting that VGLUT2 is causally contributing to DA neuron resilience.15,17 In addition, some evidence suggests that surviving DA neurons can upregulate VGLUT2 expression as part of a compensatory response to acute toxic insult.15,17,18 However, while DA neuron-selective toxicants are powerful tools for modelling the consequences of DA neuron loss in Parkinson’s disease, they have more limited utility to model its causes, and it has remained unclear how these aforementioned findings are relevant to DA neuron loss that occurs in Parkinson’s disease.
Here, we provide evidence that VGLUT2+ DA neurons are resilient not only in response to neurotoxins but also to chronic α-synuclein stress. Further, we discovered that VGLUT2+ DA neurons are greatly enriched in surviving DA neurons from human Parkinson’s disease patients. These results are consistent with a neuroprotective role for endogenous VGLUT2 expression in DA neuron survival in Parkinson’s disease.
Materials and methods
Animals
We used adult C57BL/6J wildtype mice or mice expressing Cre recombinase under control of the dopamine transporter. More details can be found in the Supplementary material. All mice were used in accordance with protocols approved by the University of California, San Diego (UCSD).
Human brains
De-identified human tissue blocks were obtained from NIH NeuroBioBank, the University of Pennsylvania and UCSD. See Supplementary material for detailed information.
Stereotaxic injections
6-OHDA was infused into the medial forebrain bundle and preformed fibrils (PFF) of α-synuclein into the dorsal striatum. Adeno-associated viruses (AAV) expressing eGFP or human α-synucleinA53T were injected into the SNc. See Supplementary material for detailed information.
Preformed fibrils of α-synuclein
Mouse α-synuclein PFF were prepared as described previously.19 See Supplementary material for detailed information.
Immunohistochemistry
Mice were perfused with 4% paraformaldehyde and coronal sections stained with indicated antibodies. See Supplementary material for detailed information.
Fluorescent mRNA in situ hybridization in mouse brain
Sections were stained using the multiplex fluorescent RNAscope assay (Advanced Cell Diagnostics). See Supplementary material for detailed information.
Chromogenic mRNA in situ hybridization in human brain
Tissue sections were stained using a modified protocol of the RNAscope Duplex Assay. See Supplementary material for detailed information.
Neuron counting
For RNAscope, a neuron was deemed positive for a given mRNA if at least four puncta were present in close proximity to a DAPI-labelled nucleus. Four sections (covering the midbrain and spaced approximately 100–150 μm) from at least four animals were counted. For human RNAscope, one section per brain was counted. All imaging and counting were performed by an experimenter blind to treatment/group.
Statistics
GraphPad Prism (GraphPad Software Inc., San Diego, CA) was used to analyse data and create graphs. All data are expressed as means ± SEM.
Data availability
The authors confirm that the data of this study are available within the article, in its Supplementary material and/or from the corresponding authors on reasonable request.
Results
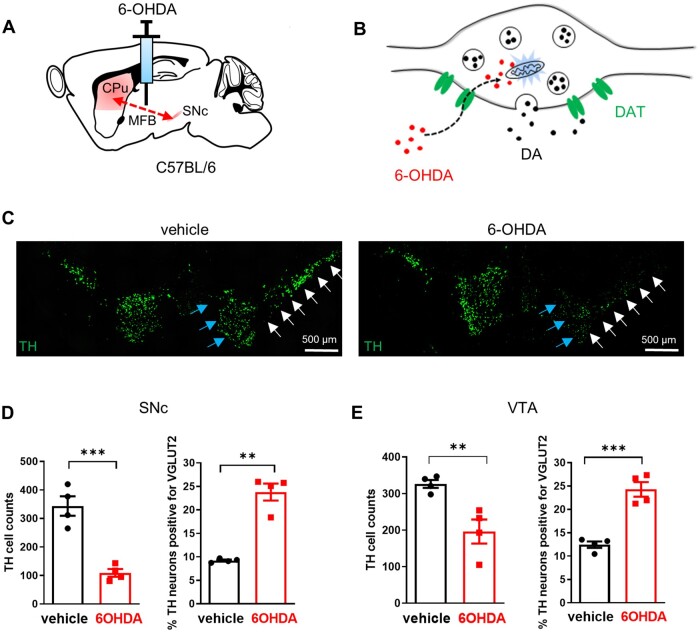
Because VGLUT2 protein localizes primarily to axon terminals, detecting (or failing to detect) its presence in neuronal soma (e.g. by immunohistochemistry) is not a reliable indicator of whether a neuron has a glutamatergic phenotype.20 We therefore relied on multiplex mRNA in situ hybridization to identify if DA neurons in SNc or VTA have altered levels of Slc17a6 (VGLUT2) expression following injury. We first made use of 6-hydroxydopamine (6-OHDA), which acutely induces robust DA neurodegeneration. We injected 6-OHDA into the medial forebrain bundle (Fig. 1A), which causes both retrograde and anterograde degeneration of DA neurons (Fig. 1B), and allows for comparison of SNc and VTA neurons that both traverse the medial forebrain bundle. 6-OHDA reduced the number of neurons expressing the DA marker tyrosine hydroxylase (TH) by 68 ± 3.9% in the SNc, whereas VTA DA neurons were reduced by only 40 ± 10.1%, confirming that VTA is more resilient to this insult (Fig. 1C–E). Co-labelling for VGLUT2 revealed that the number of TH+/VGLUT2− neurons counted was significantly decreased (SNc: control: 309 ± 31.6; 6-OHDA: 84 ± 13.5; VTA: control: 282 ± 12.8; 6-OHDA: 146 ± 28.0), whereas the number of TH+/VGLUT2+ neurons counted was unchanged after 6-OHDA lesion (SNc: control: 34 ± 3.1; 6-OHDA: 25 ± 0.9; VTA: control: 45 ± 3.8; 6-OHDA: 51 ± 5.5; Supplementary Fig. 1). Accordingly, when expressed as a ratio, the fraction of DA neurons co-positive for VGLUT2 was approximately doubled in both SNc and VTA (Fig. 1D and E), suggesting that VGLUT2+ DA neurons are more resilient to degeneration, and/or that VGLUT2 expression is increased in response to injury.
Figure 1.
Medial forebrain bundle 6-OHDA increases the fraction of DA neurons expressing VGLUT2 in both SNc and VTA. (A) Schematic of unilateral medial forebrain bundle (MFB) 6-OHDA injection leading to anterograde and retrograde degeneration of midbrain DA neurons. (B) Cartoon depicting selective 6-OHDA (red circles) uptake via the DA transporter (DAT, green) into DA varicosities where it induces rapid degeneration through oxidative stress and mitochondrial disruption. (C) Example images showing coronal sections with TH+ midbrain DA neurons from vehicle- and 6-OHDA-injected animals. Note reduction in TH mRNA signal in both VTA (blue arrows) and SNc (white arrows) of 6-OHDA treated mouse (right panel). (D) DA neuron counts are reduced, but the fraction of DA neurons that express VGLUT2 is increased in SNc and (E) in VTA; **P < 0.01; ***P < 0.001, two-tailed unpaired t-test.
The acute 6-OHDA model we employed does not recapitulate the slow, progressive degeneration or intracellular inclusions characteristic of Lewy body pathology typical of idiopathic Parkinson’s disease. Because mutations in α-synuclein are a common cause of familial Parkinson’s disease and α-synuclein aggregates are a major constituent of Lewy bodies,21 we next tested the effects of two distinct α-synuclein models on VGLUT2 expression in DA neurons.
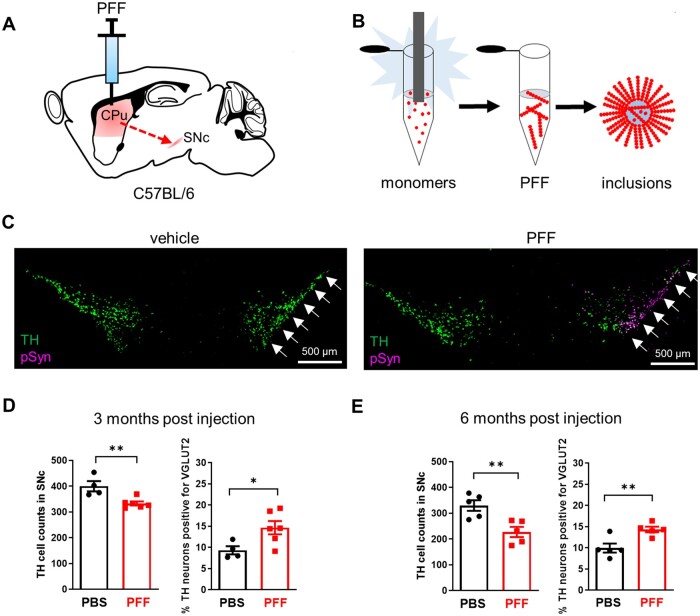
We first injected PFF of α-synuclein unilaterally into the dorsal striatum of C57Bl/6 mice (Fig. 2A). PFFs induced misfolding and aggregation of endogenous α-synuclein and the formation of Lewy body-like intracellular inclusions (Fig. 2B and Supplementary Fig. 2B and D), followed by slow progressive degeneration of distal DA neuron terminals and cell bodies in the SNc over several months.22 Consistent with prior results, we found fewer TH+ SNc DA neurons in the PFF groups compared to vehicle controls at both 3 and 6 months after injection (Fig. 2C–E). Co-labelling for VGLUT2 revealed fewer TH+/VGLUT2− neurons following PFF exposure (3 months: PBS: 336 ± 29.8, PFF: 281 ± 6.0; 6 months: PBS: 295 ± 20.5, PFF: 192 ± 16.9), but no change in the number of TH+/VGLUT2+ neurons (3 months: PBS: 36 ± 3.4, PFF: 51 ± 6.8; 6 months: PBS: 35 ± 2.9, PFF: 35 ± 3.4) (Supplementary Fig. 2A and C). Hence, VGLUT2 was more frequently observed in surviving DA neurons at both time points (Fig. 2D–E), and the levels of VGLUT2 expression per VGLUT2+ DA neuron were also increased (Supplementary Fig. 2D), suggesting that VGLUT2+ DA neurons were more resilient and that α-synuclein stress led to a transcriptional upregulation of VGLUT2.
Figure 2.
VGLUT2 expression emerges in response to α-synuclein fibril pathology. (A) PFF of α-synuclein injected ipsilaterally into striatum leads to accumulation of α-synuclein aggregates in distal SNc DA neurons. (B) Purified recombinant α-synuclein fibrillized by sonication induces synuclein aggregation in vivo. (C) Three months after unilateral injection, co-labelling for TH mRNA and Ser-129 phosphorylated α-synuclein (pSyn) protein in coronal sections through SNc revealed reduced TH signal concomitant with pSyn labelling in PFF- but not vehicle-treated mice. (D) DA neuron counts in SNc are reduced, but the fraction of DA neurons expressing VGLUT2 is increased compared to vehicle controls, at both 3 months and (E) 6 months following PFF exposure; *P < 0.05; **P < 0.01, two-tailed unpaired t-test.
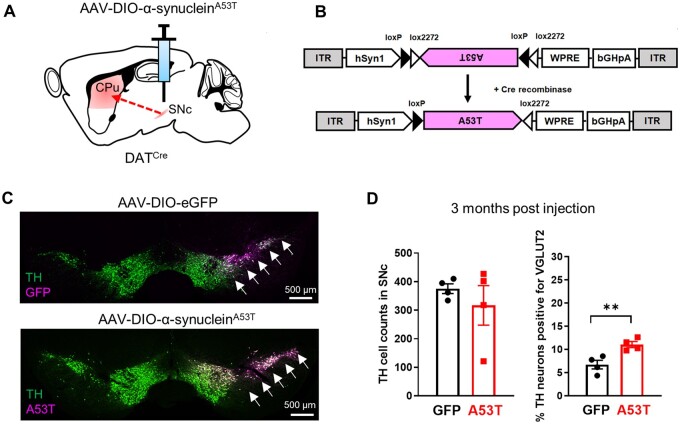
To validate this finding, we tested a second model of α-synuclein stress that relies on AAV-mediated overexpression. Previous studies have shown virus-based overexpression of α-synuclein can induce DA neuron degeneration and dysfunction over several months.23,24 We thus generated AAV vectors to drive cell-type specific expression of the disease-associated A53T variant of human α-synuclein,25 or GFP control, in the SNc of mice expressing Cre recombinase in DA neurons (Fig. 3A–C and Supplementary Fig. 3B). We did not detect significant DA neuron loss 3 months after α-synucleinA53T overexpression (TH+/VGLUT2−: GFP: 348 ± 12.7; A53T: 275 ± 60.4; TH+/VGLUT2+: 27 ± 4.3; A53T: 42 ± 10.3; Fig. 3D and Supplementary Fig. 3C). Nonetheless, we observed a significant increase in the fraction of DA neurons that expressed VGLUT2 (Fig. 3D and Supplementary Fig. 3D), again suggesting that VGLUT2+ DA neurons conferred resilience and/or that α-synuclein stress induced transcriptional upregulation of VGLUT2.
Figure 3.
Emergence of VGLUT2 without DA neuron loss after heterologous expression of human α-synucleinA53T. (A) Strategy for unilateral expression of AAV-DIO-human α-synucleinA53T in the SNc of DATCre mice. (B) AAV construct showing that in the presence of Cre recombinase, α-synucleinA53T is recombined to allow for cell-type-specific expression. (C) Immunohistochemistry shows expression of GFP or human α-synucleinA53T, detected in TH+ DA neurons in coronal sections through SNc using the human α-synuclein-specific antibody 15G7. (D) SNc DA neuron counts were not significantly altered 3 months after heterologous expression of α-synucleinA53T (left), but the fraction of DA neurons expressing VGLUT2 was increased (right); **P < 0.01, two-tailed unpaired t-test.
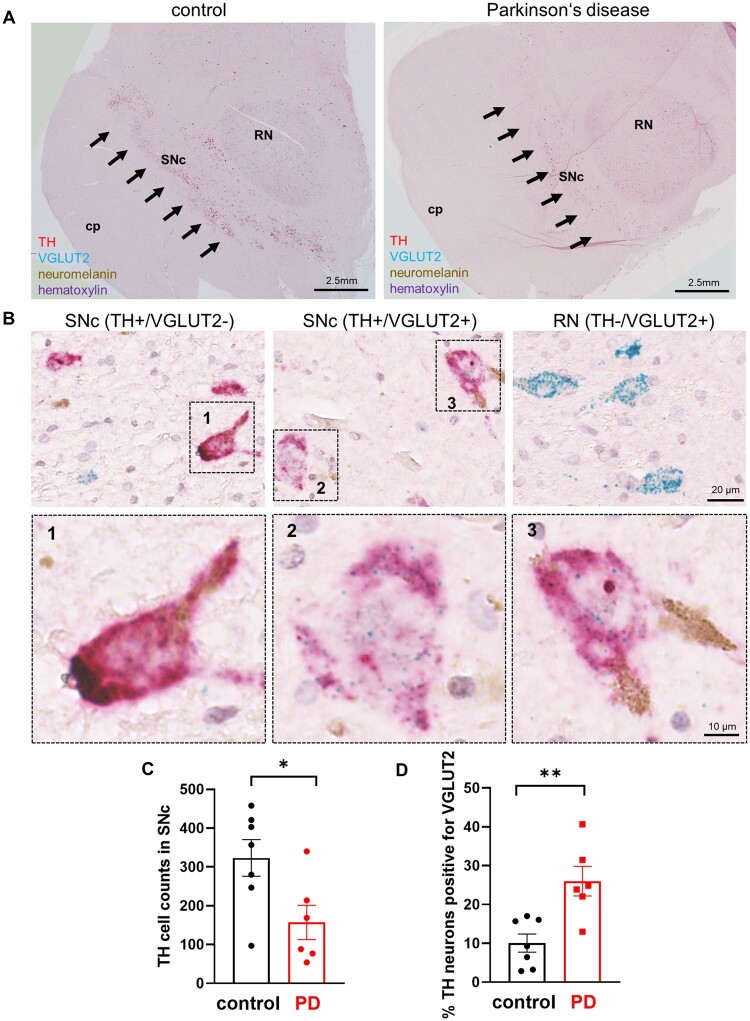
While these data indicate that VGLUT2 was increased in response to α-synuclein stress and are consistent with our previous work demonstrating that VGLUT2 is neuroprotective in DA neurons,15 the findings do not directly address relevance to human Parkinson’s disease. To test this, we developed a multiplex chromogenic assay to detect the co-localization of mRNA encoding TH (diffuse magenta signal) and VGLUT2 (blue punctate signal) in the SNc (Fig. 4A, B and Supplementary Fig. 4A) of Parkinson’s disease patients and age-matched controls (Supplementary Table 1 and Supplementary Fig. 4C and D), TH+ neurons were abundant in the neuromelanin-rich SNc and densely labelled VGLUT2+ neurons were readily apparent throughout the red nucleus (Fig. 4A, B and Supplementary Fig. 4A), and the number of SNc TH+ neurons were reduced in Parkinson’s disease (Fig. 4C) establishing the validity of our detection assay. We also identified TH+ neurons in SNc that expressed VGLUT2 at levels far above background, but at levels much lower than observed in the red nucleus (Fig. 4B and Supplementary Fig. 4A). Similar to our mouse studies, we observed a significant decrease in the number of TH+/VGLUT− neurons in Parkinson’s disease patients (control: 293 ± 30; Parkinson’s disease: 110 ± 28.3), but no reduction in the number of TH+/VGLUT2+ neurons counted (30 ± 8.1; Parkinson’s disease: 47 ± 16.7) (Supplementary Fig. 4B). Also comparable to our findings in mice, we found 10 ± 2.3% of SNc DA neurons were positive for VGLUT2 in human control participants not diagnosed with Parkinson’s disease or another synucleinopathy; but detected a much higher fraction of DA neurons that expressed VGLUT2 (26 ± 3.8%) in the SNc of participants that had been diagnosed with Parkinson’s disease (Fig. 4D). These data provide a direct link between VGLUT2 expression and DA neuron resilience in human Parkinson’s disease.
Figure 4.
VGLUT2+ DA neurons are enriched in SNc of human Parkinson’s disease. (A) Widefield view of transverse section containing SNc from control and Parkinson’s disease participants with chromogenic labelling for mRNAs encoding TH (diffuse magenta) and VGLUT2 (blue puncta) counter-stained with haematoxylin; brown signal is neuromelanin; cp = cerebral peduncle; RN = red nucleus. (B) Higher magnification images of SNc DA neurons expressing TH without VGLUT2 (left), expressing TH and VGLUT2 (middle), or RN neurons expressing VGLUT2 without TH (right). (B1–3) Inset images zoomed on boxes as depicted from B. (C) Reduced SNc DA neuron counts in sections from Parkinson’s disease patients; (D) but an increased fraction of SNc DA neurons expressed VGLUT2 in Parkinson’s disease compared to age-matched control participants not diagnosed with Parkinson’s disease; *P < 0.05; **P < 0.01, two-tailed unpaired t-test.
Discussion
Our findings provide new support for VGLUT2 in DA neuron resilience, not only after neurotoxin exposure, but also on α-synuclein stress and in human Parkinson’s disease. Using different mouse models of DA neuron injury, we demonstrate that the fraction of VGLUT2+ DA neurons is elevated in response to the neurotoxin 6-OHDA and in two different models of α-synuclein pathology. Moreover, we obtained evidence that the increased fraction of VGLUT2+ DA neurons is also observed in post-mortem SNc of Parkinson’s disease patients. Together, our findings indicate that neurotransmitter cotransmission (i.e. VGLUT2 expression) may confer increased resilience in the pathogenesis of a human neurodegenerative disease.
In previous studies, we and others have shown that VGLUT2 is expressed in subsets of midbrain DA neurons, that endogenous VGLUT2 is neuroprotective, that VGLUT2 expression in DA neurons is dynamically regulated across development and that it may re-emerge in response to acute chemical insult.15–18,26 However, previous studies relied exclusively on acute neurotoxin exposures that may little relate to intrinsic mechanisms of degeneration in Parkinson’s disease. In our current study, we addressed the limited face-validity of these toxicant-based Parkinson’s disease models by using two different α-synuclein pathology models. Although no model perfectly mimics human Parkinson’s disease, these α-synuclein models reflect key aspects of the human condition including progressive DA neurodegeneration and accumulation of α-synuclein+ intracellular inclusions.
The PFF model has the advantage that its intracellular inclusions are reminiscent of human Lewy bodies and can propagate through the nervous system, consistent with prion-like properties of fibrillized α-synuclein.22 On the other hand, the α-synuclein overexpression model mimics familial Parkinson’s disease cases where increased α-synuclein gene dosage impairs cellular function and leads to Parkinson’s disease symptoms and pathology.27 In both α-synuclein models, we found that the fraction of VGLUT2+ DA neurons was elevated after exposure, while VGLUT2+ DA neurons were spared. Additionally, in line with our previous work and work by others using neurotoxins, we found that α-synuclein stress also led to transcriptional upregulation of VGLUT2 in surviving DA neurons.15–18,26
Similar results were obtained in human post-mortem brains of Parkinson’s disease patients suggesting that VGLUT2 in DA neurons may confer resilience against neurodegeneration, represent a new therapeutic target or be a useful neuropathological biomarker. This may be particularly true if VGLUT2 is transiently increased early in neuronal degeneration, which could help to distinguish prodromal Parkinson’s disease from normal ageing processes. Although present tools limit the utility for VGLUT2 to serve as a Parkinson’s disease biomarker, this may change with technical advances in the future.
Together, our experiments suggest that VGLUT2 confers neuroprotection to DA neurons. This is in line with previous work demonstrating that genetic deletion of VGLUT2 from DA neurons increased DA neuron susceptibility to neurotoxins and therefore argues for a causal role of VGLUT2 in DA neuron resilience.15,17 We hypothesize that VGLUT2 expression could promote resilience because it can increase the vesicular sequestration of DA or other reactive substrates for the vesicular monoamine transporter (VMAT2). This is because when VGLUT2 and VMAT2 sort to the same vesicle, glutamate co-entry can increase the pH gradient across the vesicle membrane and thereby synergize DA filling.11,12 Indeed, evidence indicates that with increased activity or metabolic demand there is increased VGLUT-mediated acidification of DA-containing vesicles.13 In addition, recent findings suggest that glutamate released by SNc DA neurons has privileged access to metabotropic glutamate receptors on striatal cholinergic interneurons,28,29 and that changes in the activation of these receptors may contribute to early deficits in Parkinson’s disease.30 However, there is also evidence that sustained or high levels of VGLUT2 expression is uniquely toxic to SNc DA neurons,15,26 perhaps explaining why endogenous VGLUT2 levels in DA neurons are kept very low. Therefore, therapeutic strategies that enhance VGLUT2 function (e.g. through allosteric modulation) without increasing VGLUT2 expression may prove fruitful in preventing DA neuron loss in Parkinson’s disease.
Supplementary Material
Acknowledgements
We are grateful to NIH NeuroBioBank (University of Miami, Miller School of Medicine, Brain Endowment Bank) for providing some of the human post-mortem brains used in this study.
Funding
Funding was provided by NIH K99-AG059834 (T.S.), R01-DA036612 (T.S.H.), P30-AG062429 (R.A.R.), U19-AG062418 (E.B.L. and J.Q.T.) and the Austrian Science Fund J3656-B24 (T.S.).
Competing interests
The authors have no conflict of interest.
Supplementary material
Supplementary material is available at Brain online.
Abbreviations
- DA
dopamine
- PFF
preformed fibrils
- SNc
substantia nigra pars compacta
- VTA
ventral tegmental area
References
- 1. Dorsey ER, Elbaz A, Nichols E, et al. . Global, regional, and national burden of Parkinson's disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olanow CW. The scientific basis for the current treatment of Parkinson's disease. Annu Rev Med. 2004;55:41–60. [DOI] [PubMed] [Google Scholar]
- 3. Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci. 2017;18(2):101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Poulin JF, Caronia G, Hofer C, et al. . Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018;21(9):1260–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirsch EC, Graybiel AM, Agid Y. Selective vulnerability of pigmented dopaminergic neurons in Parkinson's disease. Acta Neurol Scand Suppl. 1989;126:19–22. [DOI] [PubMed] [Google Scholar]
- 6. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114(5):2283–2301. [DOI] [PubMed] [Google Scholar]
- 7. Gibb WR, Lees AJ. Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1991;54(5):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trudeau LE, Hnasko TS, Wallen-Mackenzie A, Morales M, Rayport S, Sulzer D. The multilingual nature of dopamine neurons. Prog Brain Res. 2014;211:141–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Root DH, Wang HL, Liu B, et al. . Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Sci Rep. 2016;6:30615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30(24):8229–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hnasko TS, Chuhma N, Zhang H, et al. . Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65(5):643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hnasko TS, Edwards RH. Neurotransmitter corelease: Mechanism and physiological role. Annu Rev Physiol. 2012;74:225–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aguilar JI, Dunn M, Mingote S, et al. . Neuronal depolarization drives increased dopamine synaptic vesicle loading via VGLUT. Neuron. 2017;95(5):1074–1088.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viereckel T, Dumas S, Smith-Anttila CJ, et al. . Midbrain gene screening identifies a new mesoaccumbal glutamatergic pathway and a marker for dopamine cells neuroprotected in Parkinson's disease. Sci Rep. 2016;6:35203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinkellner T, Zell V, Farino ZJ, et al. . Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J Clin Invest. 2018;128(2):774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kouwenhoven WM, Fortin G, Penttinen AM, et al. . VGluT2 expression in dopamine neurons contributes to postlesional striatal reinnervation. J Neurosci. 2020;40(43):8262–8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen H, Marino RAM, McDevitt RA, et al. . Genetic deletion of vesicular glutamate transporter in dopamine neurons increases vulnerability to MPTP-induced neurotoxicity in mice. Proc Natl Acad Sci USA. 2018;115(49):E11532–E11541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buck SA, Miranda BR, Logan RW, Fish KN, Greenamyre JT, Freyberg Z. VGLUT2 is a determinant of dopamine neuron resilience in a rotenone model of dopamine neurodegeneration. J Neurosci. 2021;41(22):4337–4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luk KC, Covell DJ, Kehm VM, et al. . Molecular and biological compatibility with host alpha-synuclein influences fibril pathogenicity. Cell Rep. 2016;16(12):3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steinkellner T, Yoo JH, Hnasko TS. Differential expression of VGLUT2 in mouse mesopontine cholinergic neurons. eNeuro. 2019;6(4):ENEURO.0161-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95(11):6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luk KC, Kehm V, Carroll J, et al. . Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338(6109):949–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kirik D, Rosenblad C, Burger C, et al. . Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22(7):2780–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45(3):939–953. [DOI] [PubMed] [Google Scholar]
- 25. Polymeropoulos MH, Lavedan C, Leroy E, et al. . Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. [DOI] [PubMed] [Google Scholar]
- 26. Buck SA, Steinkellner T, Aslanoglou D, et al. . Vesicular glutamate transporter modulates sex differences in dopamine neuron vulnerability to age-related neurodegeneration. Aging Cell. 2021;20(5):e13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eriksen JL, Przedborski S, Petrucelli L. Gene dosage and pathogenesis of Parkinson's disease. Trends Mol Med. Mar. 2005;11(3):91–96. [DOI] [PubMed] [Google Scholar]
- 28. Chuhma N, Mingote S, Yetnikoff L, et al. . Dopamine neuron glutamate cotransmission evokes a delayed excitation in lateral dorsal striatal cholinergic interneurons. eLife. 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai Y, Ford CP. Dopamine cells differentially regulate striatal cholinergic transmission across regions through corelease of dopamine and glutamate. Cell Rep. 2018;25(11):3148–3157.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cai Y, Nielsen BE, Boxer EE, Aoto J, Ford CP. Loss of nigral excitation of cholinergic interneurons contributes to Parkinsonian motor impairments. Neuron. 2021;109(7):1137–1149.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data of this study are available within the article, in its Supplementary material and/or from the corresponding authors on reasonable request.