Abstract
Introduction:
Venous thromboembolism (VTE) can cause significant morbidity and mortality in hospitalized patients, which may disproportionately occur in patients with limited mobility following spinal trauma. We aimed to characterize the epidemiology and clinical predictors of VTE in pediatric patients following traumatic spine injuries (TSI).
Methods:
We conducted a retrospective cohort analysis of children who experienced TSI, including spinal fractures and spinal cord injuries, encoded within the National Trauma Databank from 2011–2014.
Results:
Of the 22,752 pediatric TSI patients, 192 (0.8%) experienced VTE during initial hospitalization. Proportionally, more patients in the VTE group (77%) than the non-VTE group (68%) presented following a motor vehicle accident. Patients developing VTE had greater odds of presenting with moderate (OR 2.6; 95% CI 1.4–4.8) or severe Glasgow Coma Scale scores (OR 4.3; 95% CI 3.0–6.1), epidural hematoma (OR 2.8; 95% CI 1.4–5.7), and concomitant abdominal (OR 2.42; 95% CI 1.8–3.3) and/or lower extremity (OR 1.5; 95% CI 1.1–2.0) injuries. They also had greater odds of being obese (OR 2.9; 95% CI 1.6–5.5).
Neither cervical, thoracic, nor lumbar spine injuries were significantly associated with VTE. However, involvement of more than one spinal level was predictive (OR 1.4; 95% CI 1.0–1.7). Spinal cord injury at any level was also significantly associated with developing VTE (OR 2.5; 95% CI 1.8–3.5).
Patients with VTE stayed in the hospital an adjusted average of 19 days longer than non-VTE patients. They also had greater odds of discharge to a rehabilitative facility or home with rehabilitative services (OR 2.6; 95% CI 1.8–3.6).
Conclusions:
VTE occurs in a low percentage of hospitalized pediatric TSI patients. Injury severity is broadly associated with an increased odds of developing VTE; specific risk factors include concomitant injuries such as cranial epidural hematoma, spinal cord injury, and lower extremity injury. Patients with VTE also require hospital-based and rehabilitative care at greater rates than other TSI patients.
Keywords: venous thromboembolism, pediatrics, traumatic spinal injury
Introduction
Venous thromboembolisms (VTEs) comprise deep venous thrombosis (DVT), wherein blood clots in the deep veins of the extremities, and subsequent embolic events. The major cause of morbidity and mortality from VTE occurs when the embolus lodges in the pulmonary circulation as a pulmonary embolus; previous studies estimate that 23–46% of VTEs in adult patients were pulmonary emboli.1, 2 In the United States, roughly 14% of pediatric VTEs were identified as pulmonary emboli.3 Risk factors for DVT, and therefore VTE, fall generally under the categories of Virchow’s triad: hypercoagulability, circulatory stasis or turbulence, and endothelial damage.2, 4 VTE may occur disproportionally more in patients with limited mobility, which promotes circulatory stasis; common causes of limited mobility include obesity, post-operative recovery, and lengthy airline travel.5
Pediatric VTE is a rare event, with fewer than 5 cases per 100,000 children under 15 years old.6, 7 The risk of first-time VTE increases nearly exponentially with age.7 Major trauma is known to increase the risk of VTE development in both children and adults; the incidence of post-traumatic VTE is much lower in pediatric populations.5, 8–10 General risk factors associated with VTE development in pediatric trauma patients have been identified; however, little data exists regarding factors that increase VTE risk after traumatic spinal injury (TSI) specifically.8, 9, 11–13
This study aimed to assess the incidence of VTE among pediatric TSI patients, risk factors and protective factors for VTE development, and initial hospital course and disposition for these patients.
Methods
Study Design
We used prospectively collected data from the National Trauma Data Bank (NTDB) from the years 2011 to 2014 to generate the study cohort of pediatric trauma patients. This nationwide, multicenter database contains demographic, injury, and outcome data for trauma patients at initial hospitalization. As the largest registry of trauma data drawn from a high percentage of US hospitals, the NTDB has been used extensively to study traumatic brain injury and TSI in both pediatrics and adults.14
Inclusion and Exclusion Criteria
Inclusion criteria were 1) age of less than 18 years at the time of injury; 2) a designation of deep vein thrombosis or pulmonary embolism as a complication in the NTDB; 3) an ICD-9 diagnosis code for spinal fracture and/or cord injury (Appendix 1).
Statistical Analysis
The primary outcome of interest in this study was discharge disposition, as outlined previously.15 Patients were characterized as having adverse discharge if they were discharged to a rehabilitation facility or discharged to home requiring rehabilitative services. Other discharge categories included discharge to home and discharge to hospice. We considered discharge disposition as a proxy measurement for patient functional status. Student’s two-way t-tests were used to perform descriptive statistical analysis on quantitative variables. Multivariable logistic regression models were used to calculate adjusted odds ratios (aORs) for all qualitative variables. Multivariable linear regression models were used to analyze associations with continuous quantitative outcome variables. To control for confounding effects, our analyses were adjusted for age, sex, race, ethnicity, and insurance type as listed in the NTDB. All statistical analyses were conducted using R version 3.3.3 (The R Project).
Missing Data
To account for missing data, the R “amelia” package was used to perform multiple ratio imputation via a bootstrap algorithm. We ran 10 iterations of this algorithm to generate 10 imputed datasets containing information on patient demographics, clinical characteristics, hospital course, and hospital disposition. All statistical analyses were performed on these 10 datasets.
Results
Demographics
Of 22,572 pediatric trauma patients with TSI, 192 (0.8%) developed VTE during the initial hospitalization period (Table 1). 104 (54%) of these 192 patients were male with a mean age of 15.5 years (IQR 13–17); the majority of these patients were white (69%) and not Hispanic (72%). Demographic distributions were similar between patients with TSI who did or did not develop VTE (Table 1).
Table 1: Demographics.
The overall demographic distribution was similar across patients with TSI, with or without VTE.
| Total (N = 22752) | VTE (N = 192) | Non-VTE (N = 22560) | ||||
|---|---|---|---|---|---|---|
| Age | Mean | IQR | Mean | IQR | Mean | IQR |
| 15 | 12–17 | 15.5 | 13–17 | 15 | 12–17 | |
| Sex | N | % | N | % | N | % |
| Male | 13381 | 58.8 | 104 | 54.2 | 13277 | 58.9 |
| Female | 9364 | 41.2 | 88 | 45.8 | 9276 | 41.1 |
| Unknown | 7 | 0.0 | 0 | 0 | 7 | 0.0 |
| Race | N | % | N | % | N | % |
| White | 15549 | 68.3 | 133 | 69.3 | 15416 | 68.3 |
| Black/African American | 2887 | 12.7 | 25 | 13.0 | 2862 | 12.7 |
| Asian | 341 | 1.5 | 3 | 1.6 | 338 | 1.5 |
| American Indian | 244 | 1.1 | 0 | 0 | 244 | 1.1 |
| Native Hawaiian/Pacific Islander | 60 | 0.3 | 3 | 1.6 | 57 | 0.3 |
| Other | 2536 | 11.1 | 22 | 11.5 | 2514 | 11.1 |
| Unknown | 1135 | 5.0 | 6 | 3.1 | 1129 | 5.0 |
| Ethnicity | N | % | N | % | N | % |
| Hispanic | 2962 | 13.0 | 27 | 14.1 | 2935 | 13.0 |
| Not Hispanic | 15572 | 68.4 | 138 | 71.9 | 15434 | 68.4 |
| Unknown | 4218 | 18.5 | 27 | 14.1 | 4191 | 18.6 |
| Insurance | N | % | N | % | N | % |
| Private/BCBS Insurance | 9192 | 40.4 | 67 | 34.9 | 9125 | 40.4 |
| Medicare | 63 | 0.3 | 0 | 0 | 63 | 0.3 |
| Medicaid | 6032 | 26.5 | 51 | 26.6 | 5981 | 26.5 |
| Self-Pay | 1612 | 7.1 | 11 | 5.7 | 1601 | 7.1 |
| No Fault Automobile | 2815 | 12.4 | 36 | 18.8 | 2779 | 12.3 |
| Other Government Plan | 660 | 2.9 | 5 | 2.6 | 655 | 2.9 |
| Other Non-Governmental Plan | 900 | 4.0 | 5 | 2.6 | 895 | 4.0 |
| Unknown | 1430 | 6.3 | 16 | 8.3 | 1414 | 6.3 |
Mechanism of Injury
Motor vehicle accidents were the most prevalent mechanism of injury (MOI) among TSI patients both with and without VTE (Figure 1). A larger proportion (77%) of patients with TSI and VTE had a motor vehicle accident-related mechanism of injury than patients with TSI and no VTE (68%). Injury to other/homicide attempt and injury to self/suicide attempt were found more commonly among patients with both TSI and VTE, whereas sports-related and fall-related injuries were more common amongst TSI patients who did not have VTE. A comparison of the percentage of patients presenting with each MOI between patients with and without VTE revealed no statistically significant relationship (Wilcoxon matched-pairs signed rank test; p > 0.99).
Figure 1: Mechanism of injury.

Motor vehicle accidents were the most common mechanism of injury among TSI patients both with and without VTE. A larger proportion of patients with both TSI and VTE experienced motor vehicle accident-related injury, injury to other/homicide attempt, and injury to self/suicide attempt than patients with TSI and no VTE. Sports-related and fall-related injuries were more common among TSI patients with no VTE.
Clinical Characteristics associated with VTE
Patients with a moderate GCS score (aOR 2.6, 95% CI 1.4–4.8), cranial epidural hematoma (aOR 2.8, 95% CI 1.4–5.7), and severe GCS score (aOR 4.3, 95% CI 3.0–6.1) at initial hospitalization were at significantly higher risk of developing VTE (Figure 2). Concomitant injuries were also associated with increased likelihood of VTE development, specifically lower extremity injury (aOR 1.46, 95% CI 1.06–2.0) and abdominal injury (aOR 2.4, 95% CI 1.8–3.3). Obesity was the only pre-existing condition to associate significantly with increased VTE risk during hospitalization (aOR 2.9, 95% CI 1.6–5.5).
Figure 2: Factors associated with VTE.
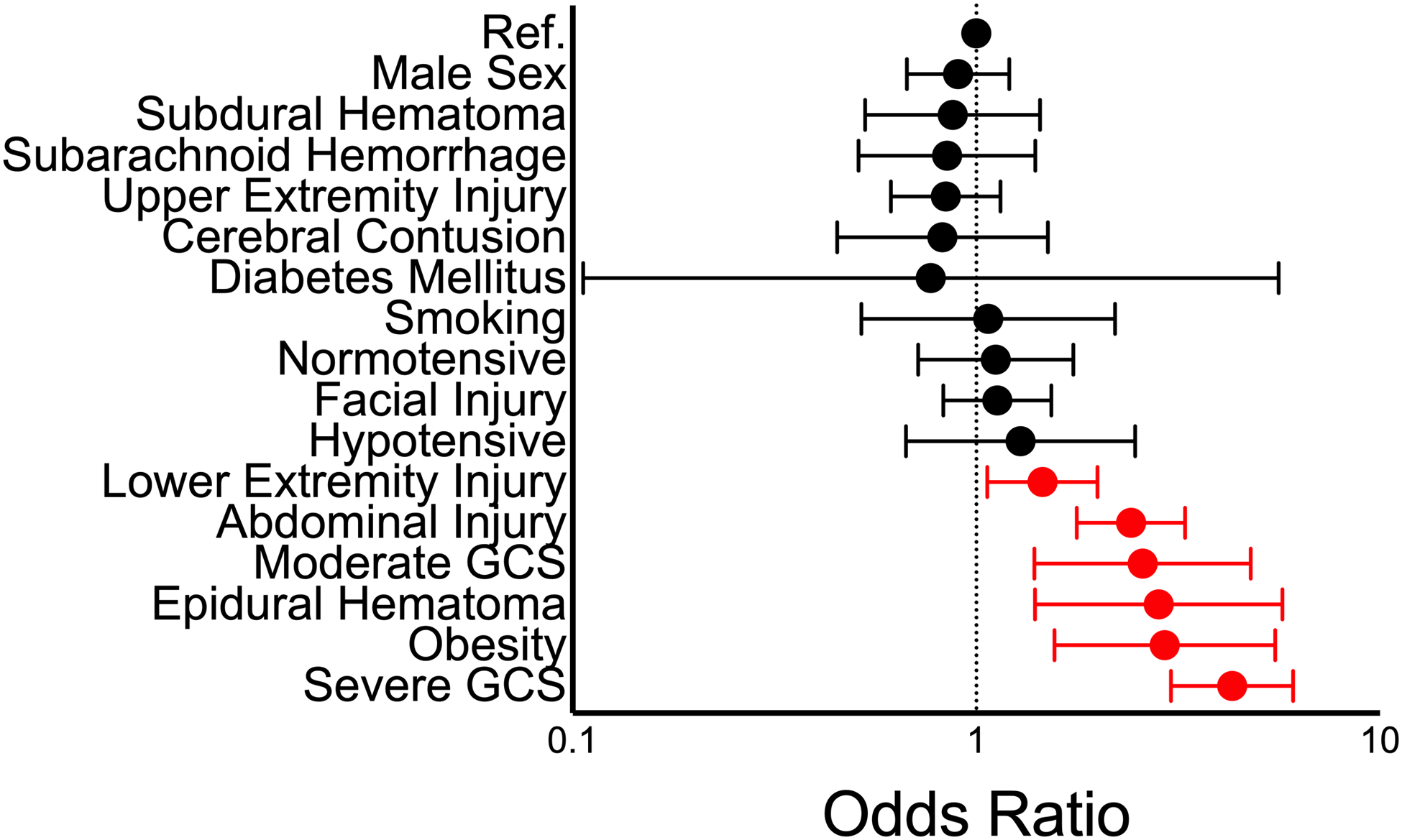
Factors correlated with increased injury severity were significantly associated with VTE development during hospitalization.
Injury to the cervical, thoracic, or lumbar spine alone at initial presentation was not significantly associated with VTE development. Conversely, patients presenting with injury involving multiple spinal levels (aOR 1.3, 95% CI 1.0 to 1.7) or involving the spinal cord (aOR 2.5, 95% CI 1.8 to 3.5) were significantly more likely to develop VTE (Figure 3).
Figure 3: Spinal injury and VTE development.
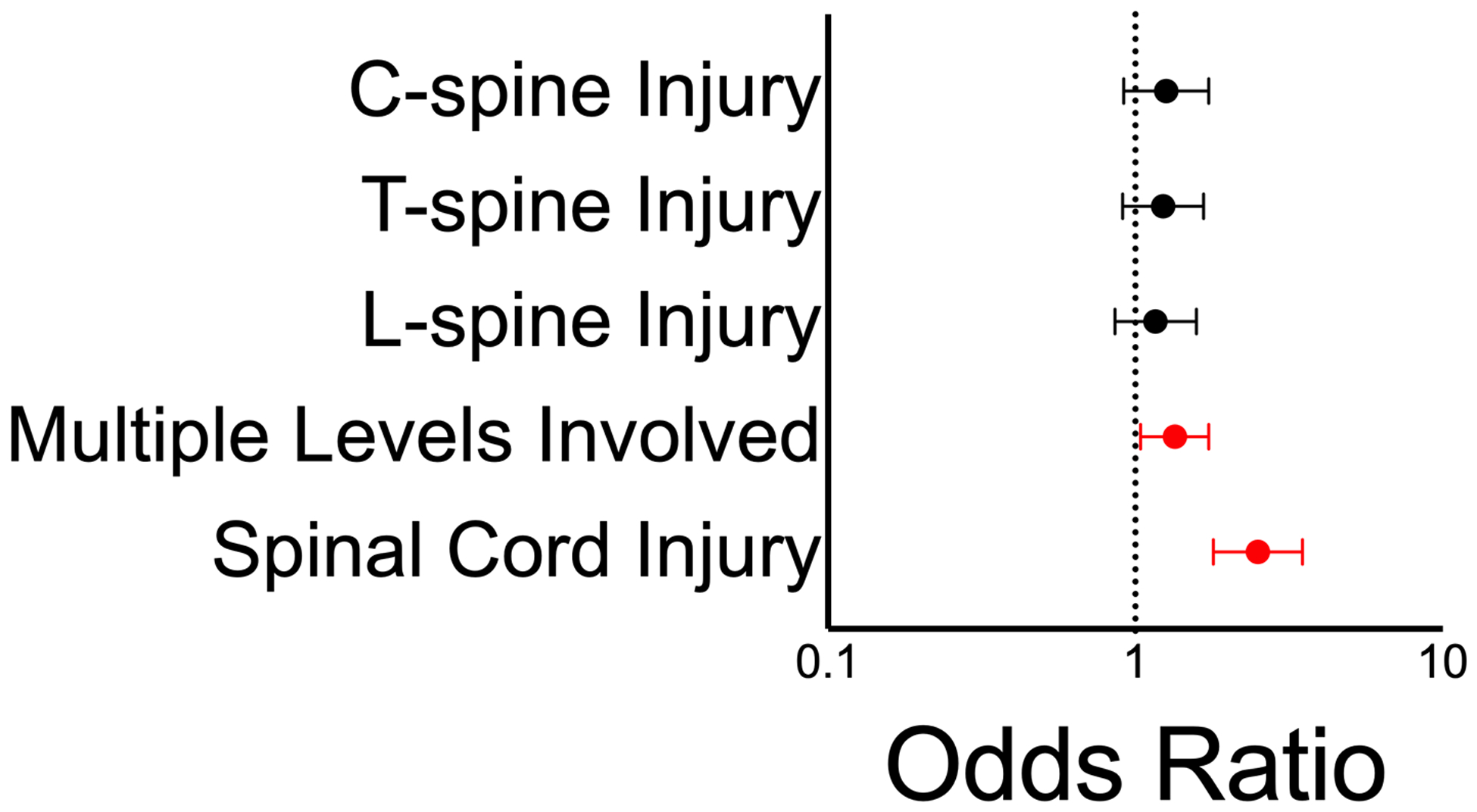
Injury at any given spinal level is not significantly associated with VTE development. Injury involving multiple spinal levels or spinal cord injury were both significantly associated with VTE development.
Hospital Course and Disposition
The length of stay among TSI patients with VTE was significantly longer than without VTE. In an adjusted model, TSI patients with VTE stayed nearly 19 days longer (mean 18.7 days, 95% CI 17.3 – 20.0 days). Developing VTE was not significantly associated with ICU stay during initial hospitalization (aOR 1.3, 95% CI 0.9–1.9). TSI patients with VTE were significantly more likely to experience adverse discharge disposition to another hospital, rehabilitation facility, or home with rehabilitative services (aOR 2.6, 95% CI 1.8– 3.6) than those without VTE (Figure 4).
Figure 4: Hospital course and disposition.

Developing VTE was not significantly associated with ICU stay during initial hospital admission. TSI patients with VTE were significantly more likely to experience adverse discharge compared to TSI patients without VTE. VTE does not appear to be associated with increased risk of death or hospice, but the relative rarity of those events may confound this effect.
Discussion
Existing studies on pediatric trauma outcomes signal a correlation between injury severity and VTE risk.12, 16, 17 Our study, which examined multi-center, nationwide data, indicates that the majority of risk factors significantly associated with VTE development in patients with TSI relate to increased injury severity, such as moderate to severe GCS and cranial epidural hematoma. In addition to limiting patient mobility, severe injury may also induce upregulation of pro-coagulative molecules to stem bleeding.18 Combined, these factors generate a pro-thrombotic state that may place trauma patients at increased risk of developing VTE. Obesity, abdominal injury, and lower extremity injury, all of which were identified as VTE risk factors in this study, could also limit patient mobility and thereby contribute to VTE development; obesity has also been previously identified as an independent risk factor for VTE in both pediatrics and adults.19–21
Although previous studies have identified smoking as a potential risk factor for VTE in adult patients with comorbidities, it was not significantly associated with VTE development here.22 Insufficient time for smoking to have increased coagulative risk could explain the lack of association in this study’s pediatric population; the NTDB does not provide pack-year data to probe this hypothesis further. Age, sex, race, ethnicity, and insurance status all were found to have no significant association with VTE development within our cohort.
While injury at any given spinal level was not significantly associated with developing VTE, multiple spinal level injury and spinal cord injury were significantly associated. The latter factors correlate with increased injury severity and may therefore be more detrimental to patient mobility, increasing the risk for VTE as described above. The spinal precautions taken to prevent worsening patient injury may also contribute to VTE risk; adults kept on prolonged spinal precautions were significantly more likely to develop VTE compared to those on shorter spinal precautions.23
Prevention of post-traumatic VTE in pediatric patients would not only decrease the clinical and financial burden associated with initial hospitalization, but would also protect against long-term morbidities associated with VTE development. While standard VTE prophylaxis protocols exist for adult patients, no universally accepted equivalent exists for pediatric patients.24 Of the independent pediatric VTE prophylaxis guidelines published in the scientific literature, all cited patient immobility and central venous catherization as primary risk factors for VTE development.25–30 The majority of these recommendations also included lower extremity trauma and obesity as risk factors, congruent with the results of this study. However, few guidelines mentioned spinal cord injury and low GCS as important risk criteria.25, 27–29 Our retrospective analysis has allowed us to identify a unique set of VTE risk factors in the pediatric TSI cohort that had not yet been fully incorporated into any one existing guideline.
Previous randomized controlled trials demonstrated that anticoagulative drugs used for VTE prophylaxis in adult patients had limited efficacy in pediatric cohorts. While our study did not specifically assess the use of pharmacologic prophylaxis in the post-trauma cohort, pediatric patients with TSI present several contraindications to anticoagulation. For instance, surgical intervention is frequently required to correct TSI; pediatric spine surgeries for non-traumatic conditions have been associated with high volumes of intraoperative blood loss that would be worsened by anticoagulative therapy.31 Moreover, while TSI patients with concurrent cranial epidural hematoma are at elevated VTE risk compared to those with no concomitant injuries, pharmacologic prophylaxis would elevate the risk of intracranial bleeding. To avoid adverse hemorrhagic events, mechanical prophylaxis (sequential compression devices, e.g.) may be a safer method of preventing VTE development in pediatric TSI patients. Future prospective analyses in this patient cohort should evaluate the efficacy of these two prophylactic approaches, optimal timing for prophylaxis initiation, and any additional VTE risk conferred by prophylaxis protocols.
The most common mechanism of injury leading to pediatric TSI both with and without VTE was motor vehicle accident. Although the number of children dying from motor vehicle accidents has decreased by 43% over the past decade, over 97,000 children under the age of 12 were injured in car accidents in 2018 in the United States alone.32, 33 According to the CDC, improper or lack of seatbelt restraint is a major risk factor driving motor vehicle accident-related injury in children.32 Children are also at increased risk for injury when they ride with a driver under the influence of alcohol or other drugs; most children injured or killed in DUI accidents are also not wearing seatbelts.34, 35 Previous initiatives have highlighted effective public health interventions that can decrease the incidence of childhood motor vehicle accident-related TSI. When certain states’ laws were modified to increase the age at which children are no longer required to ride in car booster seats, the number of children injured or killed in car-related injuries in those states decreased by 17%.36 Coordinated initiatives aimed at decreasing drunk or intoxicated driving could help lower the injury rate further, as could improved education for parents about age-appropriate car seat use.37 Among patients with VTE, injury to others/homicide attempt and falls comprised the next most frequent cause of presenting injury. The mechanisms underlying the “injury to others” category are not delineated in the NTDB; further investigation is required to identify these causes and effective intervention strategies to prevent future harm.
Clinical Significance
Strengths
By using the NTDB to generate our study’s cohort, we are able to encapsulate nationwide trends in VTE development in pediatric TSI patients. The lack of significant association between VTE development and immutable demographic factors such as age, sex, and race speaks to the generalizability of our findings. As a result, these data can be used to direct overarching changes to pediatric screening for VTE, in-patient management for pediatric TSI patients, and public policy regarding motor vehicle safety.
Limitations
The major limitation to our retrospective investigation is the inability to assess long-term prognostic information. For instance, these data may fail to capture VTEs that occur beyond the primary endpoint (i.e. after discharge), as well as factors that protect against or increase the risk of post-hospitalization VTE development. Notably, long-term follow-up of adult patients with spinal cord injury suggests that the incidence of VTE post-discharge is significantly lower than during hospitalization.38 Longitudinal study of pediatric patients with VTE indicate long-term morbidities such as post-thrombotic syndrome or persistent clot; further study is required to assess the frequency of these outcomes in the pediatric TSI cohort specifically.39 Finally, post-trauma complications may be underreported in the NTDB; our imputed datasets may therefore underestimate the true incidence of VTE between 2011–2014.40
Conclusion
VTE occurs in a low percentage of pediatric patients hospitalized with traumatic spinal injury. Injury severity, particularly spinal cord injury, is associated with likelihood of developing VTE during initial hospitalization likely due to impaired patient mobility and upregulation of pro-coagulative factors. Even after adjusting for injury severity, pediatric TSI patients with VTE experience longer hospital stays and are more likely to require rehabilitative support.
Supplementary Material
Disclosures:
The authors acknowledge support from the National Institute of General Medical Sciences T32 GM007753 (BMH).
Footnotes
Previous presentations:
This work was previously presented in virtual oral presentation format at the American Association of Neurological Surgeons 2020 Annual Meeting.
References
- 1.Cushman M, Tsai A, Heckbert S, et al. Incidence rates, case fatality, and recurrence rates of deep vein thrombosis and pulmonary embolus: the Longitudinal Investigation of Thromboembolism Etiology (LITE). Thromb Haemost. 2001;86(1) [Google Scholar]
- 2.White RH. The Epidemiology of Venous Thromboembolism. Circulation. 2003;107(23_suppl_1):I-4–I-8. [DOI] [PubMed] [Google Scholar]
- 3.Boulet SL, Grosse SD, Thornburg CD, et al. Trends in Venous Thromboembolism-Related Hospitalizations, 1994–2009. Pediatrics. 2012;130(4):e812–e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen H Pathophysiology of venous thromboembolism. 1991:250–253. [PubMed] [Google Scholar]
- 5.Cushman M and Epidemiology risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson FA, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: the Worcester DVT Study. Archives of internal medicine. 1991;151(5):933–938. [PubMed] [Google Scholar]
- 7.Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Archives of internal medicine. 1998;158(6):585–593. [DOI] [PubMed] [Google Scholar]
- 8.Cyr C, Michon B, Pettersen G, et al. Venous thromboembolism after severe injury in children. Acta Haematol. 2006;115(3–4):198–200. [DOI] [PubMed] [Google Scholar]
- 9.Vavilala MS, Nathens AB, Jurkovich GJ, et al. Risk factors for venous thromboembolism in pediatric trauma. J Trauma. 2002;52(5):922–7. [DOI] [PubMed] [Google Scholar]
- 10.Azu MC, McCormack JE, Scriven RJ, et al. Venous thromboembolic events in pediatric trauma patients: is prophylaxis necessary? J Trauma. 2005;59(6):1345–9. [DOI] [PubMed] [Google Scholar]
- 11.Andrew M, David M, Adams M, et al. Venous thromboembolic complications (VTE) in children: first analyses of the Canadian Registry of VTE. Blood. 1994;83(5):1251–7. [PubMed] [Google Scholar]
- 12.Candrilli SD, Balkrishnan R, O’Brien SH. Effect of injury severity on the incidence and utilization-related outcomes of venous thromboembolism in pediatric trauma inpatients. Pediatr Crit Care Med. 2009;10(5):554–7. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval JA, Sheehan MP, Stonerock CE, et al. Incidence, risk factors, and treatment patterns for deep venous thrombosis in hospitalized children: an increasing population at risk. J Vasc Surg. 2008;47(4):837–43. [DOI] [PubMed] [Google Scholar]
- 14.Haider AH, Saleem T, Leow JJ, et al. Influence of the National Trauma Data Bank on the study of trauma outcomes: is it time to set research best practices to further enhance its impact? J Am Coll Surg. 2012;214(5):756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S, Hauser BM, Zaki MM, et al. Morbidity after traumatic spinal injury in pediatric and adolescent sports-related trauma. J Neurosurg Spine. 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham AJ, Dewey E, Lin S, et al. Pediatric trauma venous thromboembolism prediction algorithm outperforms current anticoagulation prophylaxis guidelines: a pilot study. Pediatric Surgery International. 2020;36(3):373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truitt AK, Sorrells DL, Halvorson E, et al. Pulmonary embolism: which pediatric trauma patients are at risk? J Pediatr Surg. 2005;40(1):124–7. [DOI] [PubMed] [Google Scholar]
- 18.Martini WZ. Coagulation complications following trauma. Mil Med Res. 2016;3:35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hotoleanu C Association between obesity and venous thromboembolism. Med Pharm Rep. 2020;93(2):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: A review. Open J Prev Med. 2012;2(4):499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundbøll J, Ängquist L, Adelborg K, et al. Changes in Childhood Body‐Mass Index and Risk of Venous Thromboembolism in Adulthood. Journal of the American Heart Association. 2019;8(6):e011407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enga KF, Braekkan SK, Hansen-Krone IJ, et al. Cigarette smoking and the risk of venous thromboembolism: the Tromsø Study. J Thromb Haemost. 2012;10(10):2068–74. [DOI] [PubMed] [Google Scholar]
- 23.MacCallum KP, Kalata S, Darcy D, et al. Prolonged use of spinal precautions is associated with increased morbidity in the trauma patient. Injury. 2020;51(2):317–321. [DOI] [PubMed] [Google Scholar]
- 24.Faustino EVS, Raffini LJ. Prevention of Hospital-Acquired Venous Thromboembolism in Children: A Review of Published Guidelines. Front Pediatr. 2017;5:9–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connelly CR, Laird A, Barton JS, et al. A Clinical Tool for the Prediction of Venous Thromboembolism in Pediatric Trauma Patients. JAMA Surgery. 2016;151(1):50–57. [DOI] [PubMed] [Google Scholar]
- 26.Braga AJ, Young AE. Preventing venous thrombosis in critically ill children: what is the right approach? Paediatr Anaesth. 2011;21(4):435–40. [DOI] [PubMed] [Google Scholar]
- 27.Raffini L, Trimarchi T, Beliveau J, Davis D. Thromboprophylaxis in a pediatric hospital: a patient-safety and quality-improvement initiative. Pediatrics. 2011;127(5):e1326–32. [DOI] [PubMed] [Google Scholar]
- 28.Hanson SJ, Punzalan RC, Arca MJ, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72(5):1292–7. [DOI] [PubMed] [Google Scholar]
- 29.Meier KA, Clark E, Tarango C, et al. Venous thromboembolism in hospitalized adolescents: an approach to risk assessment and prophylaxis. Hosp Pediatr. 2015;5(1):44–51. [DOI] [PubMed] [Google Scholar]
- 30.Mahajerin A, Webber EC, Morris J, et al. Development and Implementation Results of a Venous Thromboembolism Prophylaxis Guideline in a Tertiary Care Pediatric Hospital. Hosp Pediatr. 2015;5(12):630–6. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J. 2004;13 Suppl 1(Suppl 1):S6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauber-Schatz EK, Thomas AM, Cook LJ. Motor vehicle crashes, medical outcomes, and hospital charges among children aged 1–12 years—Crash Outcome Data Evaluation System, 11 states, 2005–2008. Morbidity and Mortality Weekly Report: Surveillance Summaries. 2015;64(8):1–32. [DOI] [PubMed] [Google Scholar]
- 33.Child Passenger Safety. Centers for Disease Control and Prevention. Updated September 22, 2020. Accessed October 11, 2020. https://www.cdc.gov/injury/features/child-passenger-safety/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Ffeatures%2Fpassengersafety%2Findex.html [Google Scholar]
- 34.Quinlan K, Shults RA, Rudd RA. Child Passenger Deaths Involving Alcohol-Impaired Drivers. Pediatrics. 2014;133(6):966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Y, Liu C, Pressley JC. Child Restraint Use and Driver Screening in Fatal Crashes Involving Drugs and Alcohol. Pediatrics. 2016;138(3):e20160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eichelberger AH, Chouinard AO, Jermakian JS. Effects of booster seat laws on injury risk among children in crashes. Traffic Inj Prev. 2012;13(6):631–9. [DOI] [PubMed] [Google Scholar]
- 37.Arbogast KB, Jermakian JS, Kallan MJ, Durbin DR. Effectiveness of belt positioning booster seats: an updated assessment. Pediatrics. 2009;124(5):1281–6. [DOI] [PubMed] [Google Scholar]
- 38.Dhall SS, Hadley MN, Aarabi B, et al. Deep Venous Thrombosis and Thromboembolism in Patients With Cervical Spinal Cord Injuries. Neurosurgery. 2013;72(suppl_3):244–254. [DOI] [PubMed] [Google Scholar]
- 39.Leeper CM, Vissa M, Cooper JD, et al. Venous thromboembolism in pediatric trauma patients: Ten-year experience and long-term follow-up in a tertiary care center. Pediatric Blood & Cancer. 2017;64(8):e26415. [DOI] [PubMed] [Google Scholar]
- 40.Hemmila MR, Jakubus JL, Wahl WL, et al. Detecting the blind spot: complications in the trauma registry and trauma quality improvement. Surgery. 2007;142(4):439–48; discussion 448–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


