Abstract
Background:
Male-specific late effects after hematopoietic cell transplantation (HCT) include genital chronic graft-versus-host disease (GvHD), hypogonadism, sexual dysfunction, infertility, and subsequent malignancies, such as prostate, penile, and testicular cancer. They may be closely intertwined and cause prolonged morbidity and decreased quality of life after HCT.
Objective:
Here, we provide a systematic review of male-specific late effects in a collaboration between transplant physicians, endocrinologists, urologists, dermatologists, and sexual health professionals through the Late Effects and Quality of Life Working Committee of the Center for International Blood and Marrow Transplant Research, and the Transplant Complications Working Party of the European Society of Blood and Marrow Transplantation.
Study Design:
We utilized systematic review methodology to summarize incidence, risk factors, screening, prevention and treatment of these complications and provide consensus evidence-based recommendations for clinical practice and future research.
Results:
Most of the evidence regarding male GvHD is still based on limited data, precluding strong therapeutic recommendations. We therefore recommend to systematically screen for male genital GvHD regularly and report it to large registries to allow for a better understanding. Future research should also address treatment since little published evidence is available to date. Male-specific endocrine consequences of HCT include hypogonadism which may also affect bone health. Since the evidence is scarce, current recommendations for hormone substitution and/or bone health treatment are based on similar principles as for the general population. Following HCT, sexual health decreases and this topic should be addressed at regular intervals. Future studies should focus on interventional strategies to address sexual dysfunction. Infertility remains prevalent in patients having undergone myeloablative conditioning, which warrants offering sperm preservation in all HCT candidates. Most studies on fertility rely on descriptive registry analysis and surveys, hence the importance of reporting post-HCT conception data to large registries. Although the quality of evidence is low, the development of cancer in male genital organs does not seem more prevalent than in the general population; however, subsequent malignancies in general seem to be more prevalent in males than females, and special attention should be given to skin and oral mucosa.
Conclusion:
Male-specific late effects, probably more under-reported than female-specific complications, should be systematically considered during the regular follow-up visits of male survivors who have undergone HCT. Care of patients with male-specific late effects warrants close collaboration between transplant physicians and specialists from other involved disciplines. Future research should be directed towards better data collection on male-specific late effects and on studies about the interrelationship between these late effects, to allow the development of evidence based effective management practices.
Keywords: Survivorship, Late Effects, Male-Specific, Hematopoietic Cell Transplantation, Genital Chronic Graft-versus-Host Disease, Hypogonadism, Sexual Dysfunction, Infertility, Subsequent Malignancies
Introduction
Hematopoietic cell transplantation (HCT) is a treatment option for a variety of malignant and non-malignant disorders 1, 2. Overall survival has improved substantially over the last decades; however, HCT remains associated with considerable early and late treatment-related morbidity and mortality 3–5. It is essential for clinicians caring for transplant survivors to be aware of sex-specific complications for appropriate diagnosis, management, and counseling of patients.
Male-specific late effects include genital skin and mucosal manifestations with or without graft-versus-host disease (GvHD), hypogonadism, sexual dysfunction, infertility, and subsequent malignancies (such as prostate, penile, testicular and breast cancer). These late effects can be closely intertwined and may be under-recognized in HCT survivors. Similar to previous initiatives aimed at female survivors, 6–8 we propose a summary of guidelines for clinicians on the appropriate surveillance, management, and counseling for adult male HCT recipients, to increase awareness of these complications and to standardize practice for HCT clinicians or primary care providers. In the scope of this review, we do not address cardiovascular disease, as data suggests that while male gender is a risk factor for cardiovascular disease in general, it may not be a significant factor for developing cardiovascular disease after HCT.
Our aim is to provide an overview of male-specific late effects of adult survivors after HCT with evidence-based recommendations for clinical practice and future research. Since the approach of sex-specific late effects presents relevant differences when HCT has taken place during childhood 9, results regarding survivors who underwent transplantation during childhood or adolescence will be reported in a different manuscript.
Methods
The protocol for this review was registered at the International Prospective Register of Systematic Reviews (PROSPERO 2020 Registration ID: CRD4202014764099)10. Literature searches were conducted in MEDLINE (Ovid), Scopus, Web of Science Core Collection, PsycINFO (Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost), and the Cochrane Library databases with the assistance of a medical librarian (ES). The results were limited to articles printed in English published between January 2000 - October 2020. A base search used keywords and subject headings to describe the topics of hematopoietic cell transplantation: stem cell, bone marrow, cord blood, peripheral blood, tandem transplant; and to describe late effects: long term, delayed, sequalae, late onset, survivor, survivorship. Additional search terms were developed by each of five subgroups, including GvHD, hypogonadism, sexual health, infertility and subsequent malignancies, and the terms were combined with the base search (see Supplemental Material 1). In the GvHD subgroup specifically, the GvHD keywords were only combined with hematopoietic cell transplantation keywords and not the late effects keyword, due to low initial search results. The reference lists and citing references of relevant reviews were searched to identify additional articles that may have been missed in the database searches. Web of Science was used to search citing references.
Search results were screened and data extracted as previously described 11, 12. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) frameworks were used for assessing quality of evidence and strength of recommendation for an intervention (See Supplemental Material Table 2) 13.
The detailed study flow for each subgroup is documented in PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flowcharts (Figures 1a-1e)14. Detailed results from the different sub-groups are available in Supplemental Material Tables 3-7.
Figure 1.
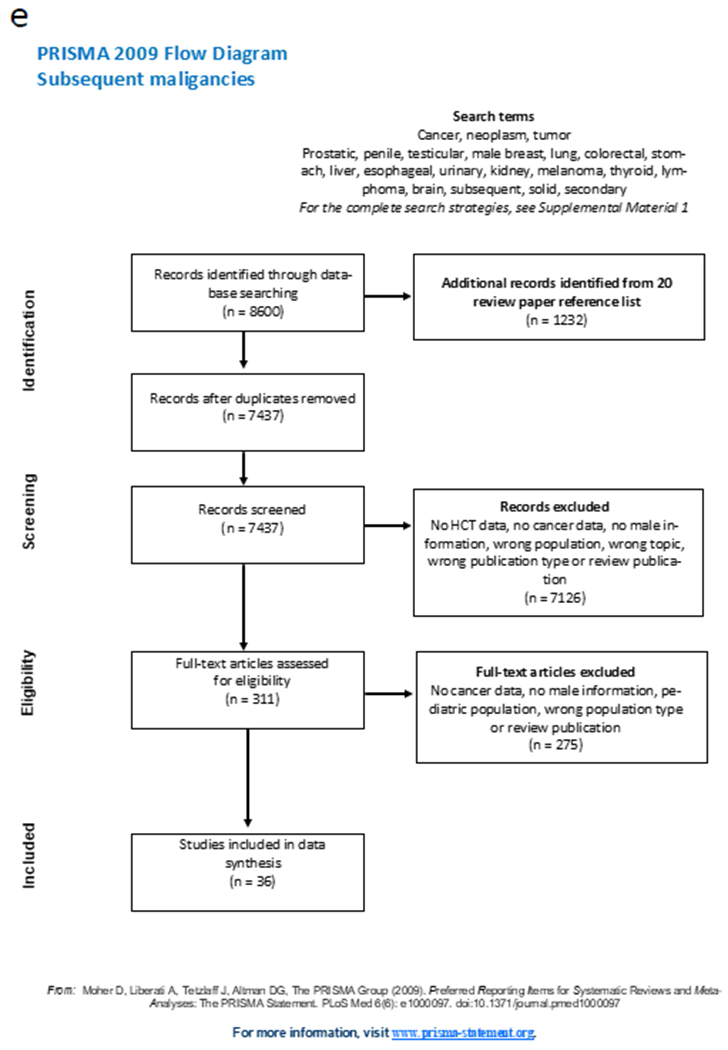
PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) flowcharts for the 5 different subgroup analysis: figure 1a, genital chronic GvHD; 1b, hypogonadism; 1c sexual dysfunction; 1d infertility; 1e, subsequent malignancies.
Abbreviations: GvHD, graft-versus-host disease; cGvHD, chronic GvHD; HCT, hematopoietic cell transplantation;
Genital chronic GvHD
In the past 10 years, several case reports15–24 and a few cohort studies25–28 have specifically reported on manifestations of genital male chronic GvHD (cGvHD), often associated with systemic cGvHD. The incidence of genital cGvHD in men varies between 5%26 and 20%25, 27, depending upon the evaluated cohort (i.e., asymptomatic patients vs patients who seek care for symptoms). The only cross-sectional cohort study to investigate the co-existence of genital lesions with cGvHD elsewhere, 3 reported a significantly higher incidence of oral, ocular, and/or cutaneous cGvHD when compared with patients without male genital lesions. The reported time of presentation in all publications exceeded two years (range 2-6 years) after transplantation (See Supplemental Material Table 3). The literature on GvHD and sexual function is relatively unclear, and will be discussed in the section on sexual dysfunction
Diagnosis
The National Institutes of Health (NIH) GvHD consensus development project was updated in 2014 to include male genital cGvHD. It now provides guidelines for diagnosis and organ scoring to determine severity 29. In male cGvHD of the genital tract, the glans penis, urethra or meatus may be affected. Symptoms may include painful intercourse or a burning sensation. Signs and diagnostic features based on NIH consensus criteria include include noninfectious inflammation with balanoposthitis, lichen sclerosis-like or lichen planus-like features, phimosis, urethral or meatal scarring or stenosis (Figure 1). Distinctive features of male genital cGvHD (which require additional evidence of cGvHD via pertinent specialist evaluation, biopsy, laboratory, imaging or other tests in the same or other organ for diagnosis) include erosions, fissures and ulcers. Contracture secondary to Peyronie’s disease (characterized by penile deformity, pain and erectile dysfunction) due to a localized, fibrotic disorder is well described16, 17, but it has not yet been integrated into the NIH diagnostic and scoring criteria of cGvHD. The NIH consensus criteria suggests organ scoring based on symptoms and genital exam, with lichen planus-like features categorized as mild, lichen sclerosis-like categorized as moderate, and phimosis or urethral/meatal scarring categorized as severe 29.
The reported cases have often been confirmed by histopathological findings, compatible with manifestations of cGvHD. Biopsies have revealed parakeratosis, focal hypergranulosis and dense lichenoid infiltrate, but also hyperkeratosis and epidermal atrophy, mild lymphocytic infiltrate and mild basal vacuolar change, dermal fibrosis and apoptotic bodies in the epithelium 15, 16, 19, 20. Histopathology is not required for confirmation of cGvHD if lichenoid changes are present, but can be performed to exclude (pre-) malignant changes and/or concomitant infections.
Screening and Treatment
Genital cGvHD manifestations can translate into genitourinary and sexual dysfunction, which can have an impact on quality of life. Moreover, genital cGvHD in males seems to be under-reported since it is seldom described in GvHD trials but affected 13% of males in a study specifically aimed at genital involvement, where it was often associated with manifestations of extragenital mucocutaneous cGvHD27. Patients often do not report genital symptoms (especially if not sexually active) and health-care providers do not specifically investigate this aspect30. Moreover, most studies to date have primarily focused on the genital issues in female recipients. Screening for genital cGvHD in male survivors should be a standard part of the routine follow-up history and physical examination on an annual basis 31. For patients with known or suspected genital cGvHD, multidisciplinary evaluation by the HCT provider, urologist and dermatologist is important to pursue the correct diagnosis (in particular to exclude subsequent malignancies) as well as suggest specific and supportive therapy. A systematic evaluation is essential and can provide a multimodal approach to treatment. Although there is no definitive consensus regarding treatment, topical and systemic immunosuppression as well as surgical intervention (circumcision for complete phimosis) have been reported (Table 2).27 More research is needed in the area of treatment for male genital cGvHD.
Hypogonadism
Hypogonadism affects approximately 15% of young male cancer survivors (age 25-45 years) 32, but there is a wide range of prevalence (6.9-84%) reported in studies of adults following both allogeneic and autologous HCT (See Supplemental Material Table 4). The prevalence has been difficult to quantify in part due to variations in definitions and lack of diagnostic criteria. Age at transplant, type of conditioning, underlying conditions and previous treatment for underlying conditions are important factors. Leydig cells, where testosterone is produced, are less vulnerable to chemotherapy and radiation compared to germinal epithelium, where spermatozoa are produced33. As such, patients may experience azoospermia and infertility, but normal testosterone levels. Patterns of Leydig cell dysfunction post-HCT include patients with elevated luteinizing hormone (LH) and normal testosterone levels (compensated hypogonadism) or elevated LH and low testosterone levels (hypogonadism)34. Recovery of hypogonadism does occur, and can sometimes be as early as within the first year after transplant34–43 . Low testosterone levels are seen in patients with acute and cGvHD after HCT, likely due to the inhibitory effects on the hypothalamic-pituitary-gonadal axis from immunosuppressive treatments such as steroids and cyclosporine.41 Iron overload, which is a common complication after HCT, is also known to lower testosterone levels in men44. In adult patients, specific symptoms of hypogonadism include loss of body hair, small testes and erectile dysfunction. Non-specific symptoms include loss of libido, anemia, fatigue, lack of motivation, reduced muscle mass and increased fat mass.44 Bone density loss is a complication of HCT survivors and can be related to hypogonadism45, immunosuppression, prolonged steroid use and/or cGvHD 46.
Diagnosis
For patients who have undergone high dose chemotherapy and/or radiation and HCT, there are insufficient data to recommend screening for gonadal dysfunction in asymptomatic patients. Guidelines for HCT survivors recommend testing and consideration of hormone replacement therapy based on symptoms, and to assess gonadal function through testing levels of follicle stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone if symptoms warrant (lack of/reduced libido or erectile dysfunction), with consideration for referral to an endocrinologist/urologist to discuss the potential risks and benefits of testosterone therapy.31
Total testosterone concentrations should be measured in the mornings when the patient is fasting. If abnormal, this should be repeated. Low testosterone levels (<250-300 mg/dL) should be confirmed, because 30% of men with initial low testosterone levels will have normal levels on repeat measurement, and because HCT survivors may recover normal testosterone levels within a year after HCT 44, 47, 48. Free testosterone levels should be determined for patients with borderline low levels, or those on glucocorticoids, as these can impact sex hormone binding globulin (SHBG) levels. Free testosterone levels should only be measured by an equilibrium dialysis method if available, otherwise calculated from total testosterone, SHBG, and albumin, using a formula that accurately reflects free testosterone by equilibrium dialysis. Reference ranges of the laboratory or formula being used should serve to determine abnormal levels, as there is no standard reference range44.
Treatment
The efficacy of testosterone replacement therapy in men with symptomatic hypogonadism in the general population has been associated with improvement in libido, erectile function, sexual activity, sexual satisfaction, areal and volumetric bone mineral density, bone strength, fat-free mass, muscle strength, as well as improvements in the positive and reductions in the negative aspects of mood49.
Guidelines for testosterone therapy and management of men with hypogonadism from the Endocrine Society Practice Guidelines and American Urologic Association have been published44, 50, though data for testosterone therapy in patients with hypogonadism and low testosterone after HCT specifically are limited31. One large randomized, placebo-controlled study of testosterone replacement in young male cancer survivors between 25 and 50 years (including a minority of HCT recipients) with low or borderline low testosterone showed that testosterone replacement therapy improved body composition with a reduction in fat mass and increase in lean body mass51. Another randomized controlled trial in men who received cytotoxic chemotherapy, including 14 patients who underwent HCT, who had compensated hypogonadism (elevated LH and low/normal testosterone level) but no overt hypogonadism, showed that testosterone replacement improved fatigue and lowered low density lipoprotein (LDL), but had no effect on bone mineral density, quality of life, body composition, or other lipid levels (Table 2).52 In addition, Combination testosterone and sildenafil treatment in this patient population has been shown to improve erectile dysfunction53
Risks of testosterone replacement therapy need to be considered and balanced with the potential benefits. Testosterone replacement may induce polycythemia, in which case the dose may need to be reduced with close follow up of the patient49. It may decrease spermatogenesis as it silences the hypothalamus-pituitary-gonadal axis, but this will usually recover if testosterone is stopped49. According to the Endocrine Society Clinical Practice Guideline, testosterone should not be given to patients with prostate cancer, with palpable nodule or induration, a prostate-specific antigen level >4 ng/mL or >3 ng/mL combined with a high risk of prostate cancer (e.g., African American or men with a first-degree relative with prostate cancer), or elevated hematocrit 44. In the previously mentioned randomized controlled trials of testosterone therapy in cancer survivors and those who underwent cytotoxic chemotherapy, skin reaction was the most common side effect, while heartburn/indigestion, chest pain, arterial hypertension, fatigue, insomnia, and anxiety were rare; there were no reported urinary symptoms or prostatic effects51, 52. Detailed information about toxicities and monitoring practices for men on testosterone replacement are available in published guidelines and reviews 44.
Hypogonadism should be treated in patients with premature gonadal failure; however, this alone will not prevent bone loss54. Therefore, standard screening and preventive practices that may include calcium, vitamin D, and bisphosphonates are warranted for patients at risk for bone loss after HCT, even in patients on testosterone replacement therapy.31 This includes discussion about preventive practices and a dual energy X-ray absorptiometry (DEXA) scan performed 1 year after HCT on all patients who underwent allogeneic HCT and earlier for those who did not have a baseline evaluation prior to transplant or those who received high dose steroids early post-HCT 31, 55. Guidelines from the American Society for Transplantation and Cellular Therapy on bone health management after HCT have been recently published and include pre-HCT evaluation recommendations as well as post-HCT guidance on bone health screening practices, vitamin supplementation, and the use of bisphosphonates and other pharmacological therapies55. Future research will need to include the impact of testosterone replacement therapy on male HCT survivors with hypogonadism, and in those with decreased bone density.
Sexual Dysfunction
Sexual difficulties occur when an individual is unable to fully engage in all phases of the human response cycle (i.e., desire, arousal, plateau, orgasm, and resolution).56 Sexual dysfunction may be diagnosed when these difficulties are accompanied by sexual distress, i.e. the psychological and emotional strain resulting from disturbances in sexual functioning.57 Sexual dysfunction following HCT is common58 (ranging from 28% to 80% depending upon domain of sexual dysfunction), as patients who undergo either autologous or allogeneic HCT tend to experience greater problems in sexual functioning than non-transplant, matched controls59 and their counterparts with similar diseases who did not undergo HCT.60, 61 Evidence suggests that sexual distress and dysfunction in men who underwent autologous versus allogeneic HCTs are comparable.28, 62–64 Additionally, in comparison to sexual function pre-HCT, satisfaction with sexual activity, ability to achieve orgasm, and frequency of intercourse decrease after HCT.65 A majority of male HCT survivors report sexual dysfunction (52%) 66, often manifesting as erectile dysfunction (ED) (12%-78%) 26, 38, 66–68, loss of sexual interest/decreased libido (23.5-62%) 26, 38, 67, 68, and decreased enjoyment of sex (33%) 26 (See Supplemental Material Table 5).
Sexual difficulties in patients with cGvHD have been described but the literature remains unclear. Some studies have suggested an association between sexual dysfunction and the presence of GvHD26, 28, 69, 70, but this has not been confirmed by others27, 71. One study specifically reported on gender differences and noted that less severe cGvHD was associated with higher sexual satisfaction in males70. Another study found that cGvHD was associated with sexual dysfunction in females, but not in males.72
It has been demonstrated that the effects of HCT on sexuality can be long lasting. In one study, male survivors at one year post-HCT reported increased concerns related to perceived attractiveness (56%), erectile dysfunction (50%), issues experiencing ejaculation (33%), and orgasm (22%) compared to baseline (time of HCT), although libido improved at one year following HCT 73. At three years post-HCT, attractiveness concerns increased further (61%), whereas other sexual concerns remained similar or decreased since one-year follow-up (22% reported concerns related to ability to achieve erection, ejaculation, or orgasm). These findings of prevalent sexual health concerns were also noted in another study assessing HCT survivors who were 1-3 years post-transplant74. Notably, males stillreport lower sexual function compared to healthy controls67, 68, even five years post-HCT.75
Other factors are known to impact sexual functioning, such as body changes, mastering one’s own life, and experiencing changes to identity 76. In comparison to female counterparts, male HCT survivors with malignant hematological disorders have been shown to be most concerned about whether their partner(s) were still attracted to them, rather than being affected by an altered body image.76 Relationship quality can also impact sexual functioning. . Male survivors have been found to be more sexually active than female HCT survivors70, 77,but they seem to be more likely to experience decreased sexual desire and report more relationship strain related to sexual dysfunction after HCT78. Spousal caregivers of HCT survivors report a negative impact of HCT on their sexual life79,80 and also report worse marital satisfaction at six- and 12-month follow-up compared to the patients themselves (of note, this finding was limited to female caregivers of male patients).81 Additional factors such as older age, physical problems, level of education, and physical inactivity have also been associated with lower sexual satisfaction, function67, and activity68 in HCT survivors.
Screening and Treatment
Despite the prevalence of sexual dysfunction following HCT, the majority of health care professionals does not address these issues (68% among both physicians and nurses) and typically wait until the patient introduces any relevant concerns.30 In a qualitative single-center study in Canada, most HCT survivors indicated they had never been asked about sexual health, and those who had voiced their concerns felt that this had been insufficiently acknowledged or addressed.82 Most survivors are willing to receive information about sexual health following HCT (79.4%), survivors with high levels of sexual health knowledge, were being 1.91 times more sexually active than those survivors with low knowledge.83 While patients may prioritize survival over sexuality during treatment, these concerns become more important as time passes and survival becomes more of a reality.84 We recommend that male HCT survivors be screened for sexual dysfunction at routine follow-up visits by the HCT or primary care provider (Table 2) 85.
Sildenafil is effective in treating ED in HCT survivors, regardless of the contributing factors (i.e., organic and psychogenic); however, it is less effective in those with reduced libido53.. El-Jawahri et al delivered a multimodal intervention aimed at improving sexual functioning in allogeneic HCT survivors, both male and female survivors 86. The intervention consisted of monthly visits (ranging from two to six) with clinicians who 1) assessed contributors to patients’ sexual concerns, 2) educated, normalized, and empowered patients to address their sexual health needs, and 3) implemented therapeutic sexual health interventions (such as treating hormonal deficiencies or erectile dysfunction, psychoeducation, referral to a specialty sexual health clinic). All domains of sexual functioning (e.g. satisfaction, interest in sex, orgasm, and erectile function) improved significantly upon completion of the intervention, with no differences by sex. In addition to improvements in sexual function, participants experienced significant improvements in quality of life and mood (depression and anxiety) following the intervention. These findings emphasize the importance of recognizing sexual dysfunction after HCT. More research is needed in the area of effective pharmacologic and non-pharmacologic interventions for male HCT survivors with sexual dysfunction. In the meantime, following society ED guidelines which emphasize shared decision making and offering escalating therapies to achieve an erection (i.e. urethral suppositories, penile injections, vacuum erection device, penile implants) should be offered 87.
Infertility
Fertility management in adult men is less problematic than in women 88, but impaired fertility in male survivors of HCT remains a frequent late effect of chemo-radiotherapy given before transplant as treatment or conditioning (between 20%-90% depending on the conditioning regimen) 42, 89–91. Loss of fertility can be psychologically traumatic and options to preserve reproductive potential should be considered and planned before HCT92–94. Fertility preservation should occur as early as possible, ideally before initiating any therapy. Semen analysis is a pivotal examination evaluating fertility in male recipients after HCT. Since 2000, there are at least 11 publications in which semen samples were evaluated during follow-up after HCT42, 89–91, 95–101; seven of them report results of more than 30 samples, including one study with samples from 224 males90, and 15 using self-reported data (See Supplemental Material Table 6).
Prevalence and risk factors
The probability of infertility following HCT depends on the cumulative dose of radiotherapy and chemotherapy, the type of chemotherapy and the age of the patient. Favorable factors for spermatogenesis recovery are age under 25-30 years at HCT, conditioning regimen without total body irradiation (TBI), long follow-up (>5years) after HCT, absence of ongoing cGvHD, and absence of underlying malignant disease90, 91, 99.
Regarding conditioning regimens, recovery of spermatogenesis has been described after a median of 4 years in 90% of patients conditioned with cyclophosphamide alone, in 50% of patients with cyclophosphamide plus busulfan or thiotepa, and 17% of patients with cyclophosphamide plus TBI or thoracoabdominal irradiation89. A prospective study evaluating 35 semen samples one year after busulfan and cyclophosphamide conditioning regimen showed that all males had impaired spermatogenesis (91% azoospermia; 9% oligozoospermia)42. Non-obstructive azoospermia (i.e. spermatogenic dysfunction) is also particularly high after TBI-based conditioning regimen, with prevalence of up to 85%90, 91, 95, 99, 100. Of note, about 4-16% of males already had impairment of spermatogenesis before HCT25, 90, mostly due to treatment of underlying malignancy. Patients with aplastic anemia, who typically do not require chemotherapy before HCT and receive a reduced intensity regimen as conditioning, frequently show a high rate (≥50%) of fertility recovery100, 102 . Time after HCT is also an important factor, as one study showed that patients with detectable spermatozoa had longer interval since HCT (>9 years), suggesting a trend to spermatogenesis production with longer follow-up, particularly in patients younger than 25 years at transplantation91.
The harmful effect of ongoing cGvHD on spermatogenesis has been discussed in three studies90, 91, 96 , with only one demonstrating a significant negative influence on fertility90. A retrospective study by the European Society for Blood and Marrow Transplantation (EBMT) focusing on male fertility after transplantation found that independent from TBI, age over 25 years at the time of HCT and ongoing cGvHD were associated with infertility after allogeneic HCT90. However, the mechanism by which GvHD may lead to azoospermia remains unclear. In an animal model103, injury to Leydig cells correlated with an intra-testicular inflammatory response, suggesting a potential indirect effect of GvHD on spermatogenesis. In patients not conditioned with TBI, ongoing cGvHD is an adverse factor for sperm recovery.
Pregnancy and fatherhood
An important outcome to demonstrate fertility is conceiving a child. Multiple retrospective studies have reported the rates of conception for men after HCT26, 89–91, 95, 96, 98–100, 102, 104–113. With one exception102 all studies consistently showed a low rate of paternity after HCT (See Supplemental Material Table 6). The true prevalence of fertility after HCT is difficult to determine because patients’ wishes regarding fatherhood are often unreported in studies. The desire for paternity was evaluated in one study: a majority (78%) reported the wish of having a child, but only 15% of the HCT-recipients transplanted for a malignant or non-malignant disease, fathered eventually a child114 . In another study 21 males had tried to conceive, with 11 pregnancies reported, six of them assisted by in vitro fertilization26.. In a small series, of 13 male patients with severe aplastic anemia who attempted to be a father, 12 (92%) succeeded.102 The reported pregnancies and deliveries from male recipients were usually uncomplicated: no excess of miscarriage, stillbirth, or congenital malformations were reported91, 102, 106, 107, 112 .
Fertility preservation
Despite the fact that sperm banking is readily available, studies show that male patients often do not recall having been counseled about this before cancer therapy. Regarding fertility preservation utilization, one study showed that only 4.7% of men age 18-40 in a national insurance database had claims for fertility preservation counseling and/or procedures prior to transplant.121 Sperm banking should be discussed with all male patients undergoing HCT, ideally before cancer treatment is initiated92, 115 . At HCT, spermatogenesis might be already impaired due to treatment of the underlying disease (Table 2). Patients who already received chemotherapy before HCT will often fulfill the minimum criteria for semen cryopreservation, since reported pre-HCT azoospermia rates range from 16%89 to 46%116. Patients who have no sperm in the ejaculate and children may be offered testicular biopsy to obtain sperm for cryopreservation (e.g., microscopic assisted testicular sperm extraction; mTESE) 117–119. Stored spermatogonial stem cells obtained from testicular biopsy might be transplanted back to restore fertility. Remove contaminated malignant cells in stored material is a crucial step before auto-transplantation. Different techniques such as fluorescence-activated cell sorting (FACS), magnetic-activated cell sorting (MACS), selective matrix adhesion technique, and nanoparticle-targeted drug delivery to malignant cells have been evaluated in preclinical studies, but none have yet been completely successful120. Possible barriers to fertility preservation before HCT may include the perception of lack of time because of the urgency to start the transplant procedure, deficient awareness on this topic by the transplant team, underestimation of the future desire for paternity, the existence of an underlying malignancy, and issues related to the insurance coverage116, 121. The long-term care team should signal readiness to discuss issues on fertility, and if needed, to undertake further investigations and consider referral for a specialized consult 31. If appropriate, contraception counseling should be done in patients who are known to be fertile or when their fertility status is not known 31.
Subsequent Malignancies
Long-term survivors of HCT are at risk of subsequent malignancies, and subsequent solid cancers are a major cause of late treatment-related mortality.122 While risk factors for the development of subsequent solid cancers, in general, are known, little is known about male-specific considerations. Sex-related differences for subsequent solid cancers include conditions that only affect male recipients because these cancers develop in the male reproductive system (prostate, testicular, penile cancers), and cancers which are more common in men than in women (See Supplemental Material Table 7).
Cancers with a higher likelihood in men
The cumulative incidence of subsequent solid malignancies for males and females ranges from approximately 1-6% at 10 years123–125. Male sex was identified as a risk factor for the development of subsequent solid cancers in several studies: overall after both autologous and allogeneic HCT124, 126, and specifically in squamous cell carcinoma (SCC) after allogeneic HCT125, 127, melanoma after autologous HCT123, and any skin cancer after allogeneic HCT128. In one study, the increased risk of subsequent solid cancers was seen in male recipients of female donors, independent of GvHD 124. Male sex has also been associated with an increased risk of death after subsequent solid cancer among survivors of autologous HCT.129
Male recipients undergoing autologous or allogeneic HCT have a 25-fold increase in the risk of oral cavity cancer compared to the general population in a single-center, case-control study.130 In studies of allogeneic HCT survivors, male sex was associated with increased risks of SCC of the oral cavity and skin,125, 127 and skin cancer including SCC and basal cell carcinoma,128 while another study did not find significant associations131. It is unclear whether the higher incidence of skin and oral cancer in males is due to genetic causes or behavioral differences.
Male-specific cancers
Only one study of adult allogeneic and autologous HCT recipients reported the cumulative incidence of prostate cancer, which was 14% at 5 years.132 Although prostate cancer may develop as a subsequent solid malignancy after HCT, its risk of development was not significantly increased compared to the general population 123, 125, 126, 133–138. Of note, when compared to the general population, the risk of prostate cancer did not vary by the age at diagnosis, time since diagnosis, by diagnosis evaluated, or by conditioning intensity.133, 135–137 In 3 retrospective studies that included 13,925 patients who underwent allogeneic HCT, a total of 365 subsequent solid cancers were observed at a median of 6 years post HCT, out of which only 6 were prostate cancers.133, 135, 138 An increased risk of mortality in association with prostate cancer was documented when compared to the general population in one study132 but this was not confirmed by others129. The overall survival following the development of prostate cancer ranges from 69-91%, with no significant differences among allogeneic and autologous survivors.129, 132
Data on genitourinary cancers following HCT is scarce. When compared to the general population, the risk of genitourinary cancers among males is about two-fold higher among study populations focusing on adult and pediatric allogeneic HCT survivors.127 Although testicular cancer may develop as a subsequent solid malignancy after HCT, its risk of development is not significantly increased compared to the general population when considering allogeneic HCT survivors.125, 135, 136, 139 Moreover, the risk of testicular cancer when compared to the general population did not vary by age at diagnosis, time since diagnosis, by diagnoses evaluated, or by conditioning intensity.125, 135, 136 A few cases of penile cancers have been reported after HCT.125, 140 Single cases of squamous cell carcinoma have been reported in patients with genital lichen sclerosis, likely related to cGvHD.23 Penile carcinoma might be underestimated, since carcinogenesis in male genital lichen sclerosis in the general population can be as high as 10%.141
Screening and management
Male survivors of HCT have a higher risk of developing subsequent solid cancers when compared to the general population. Special attention should be paid to the development of SCC of the skin and oral cavity. Screening protocols should focus on these high-risk sites in male survivors of HCT. Male genitourinary cancers such as prostate, testicular, and penile cancers are documented, although the risk does not seem significantly elevated when compared to the general population. However, these cancers might be underreported. Therefore, general population age-specific guidelines should be followed for screening and management for male-specific subsequent malignancies. There is no consensus about using prostate-specific antigen or digital rectal examination31.
Summary and Recommendations for Research and Clinical Practice
This review summarizes the existing literature regarding the prevalence and long-term management of male-specific dysfunctions in adult patients who undergo HCT (Table 2). We note specific changes linked to GvHD, which tend to develop in long term-survivors and can be managed by local therapy and, if needed, by surgical urological interventions. Most of the evidence regarding male GvHD is still based on case-reports and limited case-series, precluding strong therapeutic recommendations. We therefore recommend to systematically screen for male genital GvHD and report it to large registries to allow for a better understanding of this HCT complication. Future research should also address treatment issues since almost no published evidence is available to date to support recommendations. Male-specific endocrine consequences of HCT concern hypogonadism which may also affect bone health; it can be addressed by hormonal substitution and/or appropriate osteopenia/osteoporosis treatment, if necessary. Again, the evidence is scarce and current recommendations are based on the same principles as for the general population. Sexual health decreases after HCT so this should be addressed as early as possible in the HCT trajectory and be screened for at regular intervals thereafter. Since only few studies have robustly investigated the best interventions to offer, future efforts should aim at comparing interventional strategies to address sexual dysfunction. Fertility management in adult males is less problematic than in females, but remains extremely prevalent when patients having undergone a myeloablative conditioning, warranting offering sperm conservation in all HCT candidates. Most studies on fertility rely on descriptive registry analysis and surveys, so we stress here the importance of reporting post-HCT conception information to large registries such as EBMT and CIBMTR. Future options include involving patients to report this directly. Further research should address fertility prospectively to identify the predictors and factors associated with fertility issues after HCT. Finally, overall subsequent malignancies seem to be more prevalent in males than females, and special attention should be given to skin and oral mucosa, including genital skin changes. The development of cancer in male genital organs does not seem more prevalent than in the general population, but the quality of evidence is low and future studies should focus on a better understanding of how to prevent and treat these cancers in HCT recipients.
We are aware that there may be obstacles in addressing male-specific issues during the clinical visit. However, the high level of consent among long-term survivors to be examined specifically for genital changes has been demonstrated in a prospective study27. By systematically addressing questions on genital abnormalities, sexual dysfunction or fertility issues, the long-term care team signals readiness to discuss these issues, and if needed, to undertake further investigations and consider referral for a specialized consult. Many issues encountered by male survivors are closely intertwined. GvHD can potentially negatively impact fertility, sexual health and increase the risk of subsequent malignancies. Similarly, the advantages of substitution treatment for hypogonadism must be balanced against the potentially increased risk of prostate cancer. Therefore, a multidisciplinary approach is essential, with early referrals to urologist, sexual health specialists, dermatologist and endocrinologist when appropriate. Such collaborative clinical practices can be standardized, implemented and audited through standard operating procedures within the quality system of accredited HCT programs 142.
On behalf of the CIBMTR Late effects and Quality of Life Committee and EBMT Transplantation Complication Working Party, we provide consensus recommendations for screening and preventive measures for adult male-specific late effects after HCT. Significant knowledge gaps remain in all areas. Future research should be directed towards better data collection on male-specific late effects, on studies about the interrelationship between these late effects, and effective management practices. Care of patients with male-specific late effects warrants close collaboration between transplant physicians, dermatologists, urologists, endocrinologists, and specialists in sexual health, to improve the care of male survivors of HCT.
Supplementary Material
Figure 2. (from Müller at al. 201327).
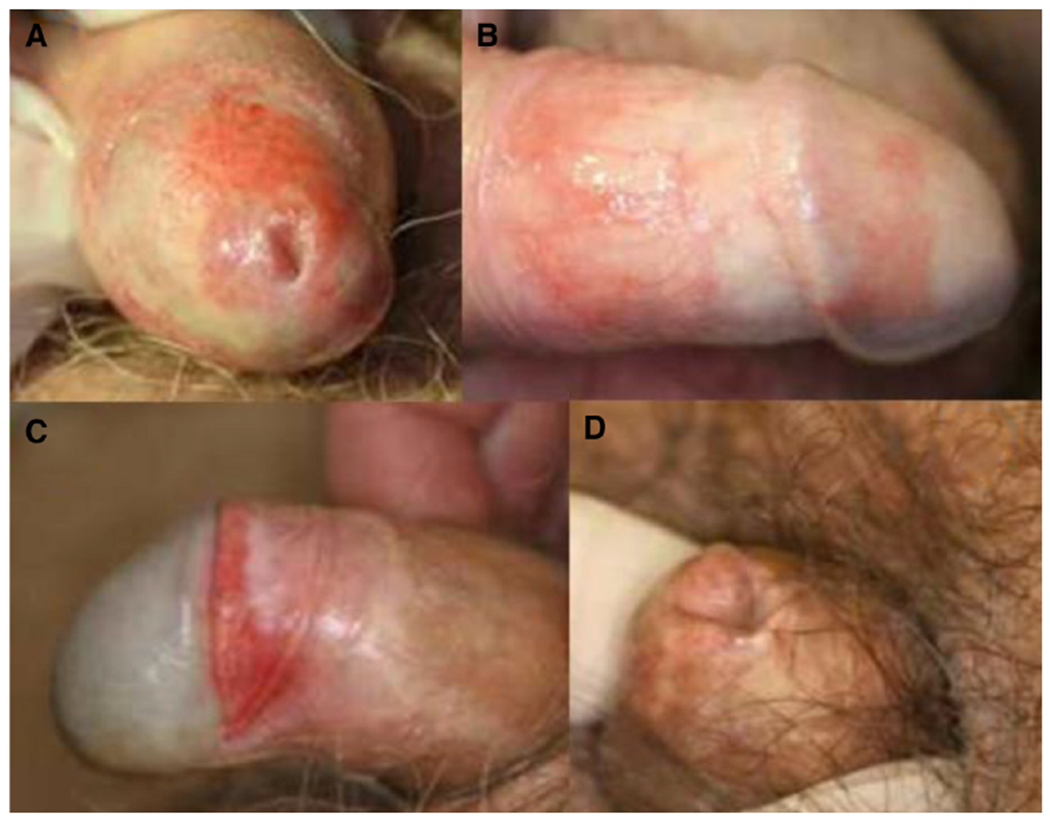
Examples of post-transplantation inflammatory genital skin changes. (A) Inflammatory (noninfectious) balanoposthitis with reddish “cayenne-pepper spots” on the glans penis and partial phimosis. (B) Shiny plaques on the glans penis and the inner layer of the foreskin. (C) Lichen sclerosis-like skin changes with erythematous and whitish sclerotic areas on the foreskin. (D) Phimosis
Table 1:
Recommendations for screening and therapy for male-specific late effects in HCT adult patients
| Potential late effects | Screening Recommendations | Therapeutic Implications | Quality of Evidence | Strength of Recommendation | Comments |
|---|---|---|---|---|---|
| Genital chronic GvHD | - Screening for symptoms (change in appearance of genitalia, a new burning sensation or painful intercourse) at each visit 27 - Genital exam, yearly 31 - Biopsy not required but can be done to exclude (pre-)malignant changes and infections |
Topical therapy (e.g. high-potency topical corticosteroids [0.05%] or calcineurin inhibitors [0.1%]) and, if necessary, systemic immunosuppression | Low | Strong | - Consider multidisciplinary evaluation by the HCT provider, urologist and dermatologist for suspected genital GvHD - The occurrence of subsequent genital skin cancer remains a concern and patients should be educated on self-examination and symptoms |
| Circumcision for complete phimosis | Low | Strong | |||
| Surgical interventions for meatal stenosis | Low | Strong | |||
| Hypogonadism | - Screening for symptoms at each visit (lack of libido or erectile dysfunction, lack of motivation, reduced muscle mass and increased fat mass) 44 - Hormonal testing (testosterone, FSH, LH), ideally done in fasting patients, first thing in the morning in patients with symptoms 31 - Low testosterone should be confirmed by a repeat test 44, 47 - Annual screening for bone loss in allogeneic HCT recipients and patients at risk of bone loss 31, 55 - DEXA scan and fracture risk evaluation at 3 months for patients without pre-transplant evaluation or if patient received high dose steroids early post-transplant |
Testosterone therapy in hypogonadal men to correct symptoms of testosterone deficiency 44 | Low | Weak | - Consider referral to an endocrinologist or urologist to discuss the potential risks and benefits of testosterone therapy - Consider toxicities of testosterone therapy: screening for polycythemia and prostate cancer before initiating treatment, and close monitoring during treatment |
| Sexual dysfunction | - Screen for sexual health regularly through the survivorship process (loss of sexual interest, concerns related to perceived attractiveness, problems obtaining erection, ejaculation or orgasm) 85 | Treatment of hormonal deficiencies, psychoeducation, referral to specialist in sexual health | Moderate | Strong | - Adapt interventions based on patient priorities - Consider referral to a psychologist for individual or couples-based interventions to address psychological contributors to sexual dysfunction |
| Sildenafil treatment, vacuum erectile device, medicated urethral system for erection, or intra-cavernous injection for erectile dysfunction | Low | Weak | |||
| Infertility | - Pre-transplant counseling about risk of infertility and fertility preservation (sperm banking offered to all adult male patients undergoing HCT) depending on the type of pretransplant therapy and the conditioning 92 - Post-transplant counseling and consideration of semen analysis |
Pre-transplant fertility preservation (sperm banking) | Moderate | Strong | - Consider discussion about alternative options for fatherhood - Consider referral to appropriate specialists for patients having difficulties conceiving 31 Contraception counseling if fertile or fertility status not known |
| Referral to reproductive health specialist for patients with infertility | Moderate | Strong | |||
|
Subsequent malignancies - Cancers only affecting men - Cancers more prevalent in men than in women |
- Cancers which affect only men (Prostate, testis and penile cancers) follow guidelines for the general population - Patients with ongoing chronic GvHD or a history of chronic GvHD should have regular full skin examination, including genitalia - Counsel patients about risk of subsequent cancers, including ones in which men are at higher risk and ones that only affect men |
Follow the guidelines for the general population | Low | Strong | - Special attention to screening for symptoms of squamous cell cancers of the skin, oral cavity and genitalia |
GvHD: graft-versus-host disease; HCT, hematopoietic cell transplantation; FSH, follicle stimulating hormone; LH, luteinizing hormone; DEXA, dual energy X-ray absorptiometry
Highlights:
Male-specific late effects after hematopoietic cell transplantation (HCT) may be closely intertwined and cause prolonged morbidity and decreased quality of life.
We utilized systematic review methodology to summarize incidence, risk factors, screening, prevention and treatment of these complications and provide consensus evidence-based recommendations for clinical practice.
Care of patients with male-specific late effects warrants close collaboration between transplant physicians and specialists from other involved disciplines.
Future research should be directed towards better data collection and studies of the interrelationship between these late effects.
Acknowledgements
The CIBMTR is supported primarily by Public Health Service U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); HHSH250201700006C from the Health Resources and Services Administration (HRSA); and N00014-20-1-2705 and N00014-20-1-2832 from the Office of Naval Research; Support is also provided by Be the Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Accenture; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies Corporation; Adienne SA; Allovir, Inc.; Amgen, Inc.; Astellas Pharma US; bluebird bio, inc.; Bristol Myers Squibb Co.; CareDx; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Eurofins Viracor; ExcellThera; Fate Therapeutics; Gamida-Cell, Ltd.; Genentech Inc; Gilead; GlaxoSmithKline; Incyte Corporation; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals, Inc.; Karyopharm Therapeutics; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Co.; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncopeptides, Inc.; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer, Inc.; Pharmacyclics, LLC; Priothera; Sanofi Genzyme; Seagen, Inc.; Stemcyte; Takeda Pharmaceuticals; Tscan; Vertex; Vor Biopharma; Xenikos BV.
Conflict of Interest
Dr. Einsele reports grants and other from BMS/Celgene, grants and other from Janssen, grants and other from Amgen, other from Takeda, grants and other from Sanofi, grants and other from GSK, during the conduct of the study. Dr. Hashmi reports other from Pfizer, other from Novartis, other from Therakos, other from Janssen, other from MSD, outside the submitted work. Dr. Inamoto reports personal fees from Novartis, personal fees from Janssen, personal fees from Meiji Seika Pharma, outside the submitted work. Dr. Phelan reports research funding from Amgen. Dr. Ross reports other from Diurnal Plc, during the conduct of the study. Dr Schoemans reports participation in advisory boards for Janssen & Novartis; speaker’s fees from Incyte, Jazz Pharmaceuticals, Takeda, Novartis and the BHS (Belgian Hematological Society); travel grants from EBMT, CIBMTR, Incyte & Gilead and research funding from Novartis & the BHS, outside of the submitted work. Dr. Rovo reports grants from Novartis, grants from CSL Behring, grants from Alexion, personal fees from Novartis, personal fees from BMS, personal fees from OrPha Swiss GmbH, other from Amgen, other from AstraZeneca, other from Sanofi, other from Celgene, outside the submitted work. Dr. Sharma reports grants from CRISPR Therapeutics, Payment or honoraria from Vindico Medical Education and personal consultancy fees from Spotlight Therapeutics and Medexus Inc., outside the submitted work. Dr. Snowden reports personal fees from MEDAC, personal fees from KIADIS, personal fees from GILEAD, personal fees from JANSSEN, personal fees from MALLINCKRODT, personal fees from JAZZ, personal fees from ACTELION, outside the submitted work. Dr. Wolff reports grants and personal fees from Novartis, personal fees from Mallinckrodt, personal fees from Behring, outside the submitted work. Dr. Gale is a consultant to Ascenage Pharma, BeiGene Ltd., Kite Pharma Inc., Fusion Pharma LLC, LaJolla NanoMedical Inc., Mingsight Parmaceuticals Inc. and CStone Pharmaceuticals; Medical Director, FFF Enterprises Inc.; Partner, AZCA Inc.; Board of Directors, RakFond Foundation for Cancer Research Support; Scientific Advisory Board, Antegene Biotech LLC and StemRad Ltd. Dr. Shah reports medical advisory board for Rocket Pharmaceuticals and Orchard Pharmaceuticals. Dr. Epperla reports being on the speaker’s bureau for Verastem and Beigene; Advisory board for Karyopharm and Honorarium for Genzyme outside of the submitted work.
Footnotes
Data Sharing
CIBMTR supports accessibility of research in accord with the National Institutes of Health (NIH) Data Sharing Policy and the National Cancer Institute (NCI) Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases de-identified datasets that comply with all relevant global regulations regarding privacy and confidentiality.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. [DOI] [PubMed] [Google Scholar]
- 2.Singh AK, McGuirk JP. Allogeneic Stem Cell Transplantation: A Historical and Scientific Overview. Cancer Res. 2016;76:6445–6451. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Armenian SH, Landier W. How I monitor long-term and late effects after blood or marrow transplantation. Blood. 2017;130:1302–1314. [DOI] [PubMed] [Google Scholar]
- 4.Inamoto Y, Lee SJ. Late effects of blood and marrow transplantation. Haematologica. 2017;102:614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tichelli A, Rovo A, Passweg J, et al. Late complications after hematopoietic stem cell transplantation. Expert Rev Hematol. 2009;2:583–601. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton BK, Goje O, Savani BN, Majhail NS, Stratton P. Clinical management of genital chronic GvHD. Bone Marrow Transplant. 2017;52:803–810. [DOI] [PubMed] [Google Scholar]
- 7.Murphy J, McKenna M, Abdelazim S, Battiwalla M, Stratton P. A Practical Guide to Gynecologic and Reproductive Health in Women Undergoing Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019;25:e331–e343. [DOI] [PubMed] [Google Scholar]
- 8.Stratton P Gynecologic care after hematopoietic cell transplantation: a call to action to include gynecologists in the transplant team. Bone Marrow Transplant. 2015;50:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Anderson L, Baker KS, et al. Late Effects Surveillance Recommendations among Survivors of Childhood Hematopoietic Cell Transplantation: A Children’s Oncology Group Report. Biol Blood Marrow Transplant. 2016;22:782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A, Badawy S, Suelzer E, et al. Male-specific late effects after hematopoietic cell transplantation. PROSPERO 2020. CRD42020147640. Aug 13, 2020 ed. [Google Scholar]
- 11.Sharma A, Badawy S, Suelzer E, et al. Systematic Reviews in Hematopoietic Cell Transplantation and Cellular Therapy: Considerations and Guidance from the American Society for Transplantation and Cellular Therapy, European Society for Blood and Marrow Transplantation, and Center for International Blood and Marrow Transplant Research Late Effects and Quality of Life Working Committee. Transplantation and Cellular Therapy. 2021:in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Badawy SM, Suelzer EM, et al. Systematic reviews in hematopoietic cell transplantation and cellular therapy: considerations and guidance from the American Society for Transplantation and Cellular Therapy, European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research late effects and quality of life working committee. Bone Marrow Transplant. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au WY, Yeung CK, Cheung MC, Trendell-Smith NJ. Penile lichen sclerosus after allogeneic stem cell transplantation. Br J Dermatol. 2008;159:470–472. [DOI] [PubMed] [Google Scholar]
- 16.Grigg AP, Underhill C, Russell J, Sale G. Peyronie’s disease as a complication of chronic graft versus host disease. Hematology. 2002;7:165–168. [DOI] [PubMed] [Google Scholar]
- 17.Jain NA, Venkatesan K, Anandi P, et al. A Rare Consequence of Chronic Graft Versus Host Disease - Peyronie’s Disease. Arch Cancer Res. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kami M, Kanda Y, Sasaki M, et al. Phimosis as a manifestation of chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1998;21:721–723. [DOI] [PubMed] [Google Scholar]
- 19.Lara LA, De Andrade JM, Mauad LM, et al. Genital manifestation of graft-vs.-host disease: a series of case reports. J Sex Med. 2010;7:3216–3225. [DOI] [PubMed] [Google Scholar]
- 20.Nylander E, Wahlin YB, Lundskog B, Wahlin A. Genital graft-versus-host disease in a male following allogeneic stem cell transplantation. Acta Derm Venereol. 2007;87:367–368. [DOI] [PubMed] [Google Scholar]
- 21.Odorici G, Baraldi C, Loi C, Bardazzi F. Chronic graft-versus-host disease of the male genitalia: an underrecognized manifestation. J Dtsch Dermatol Ges. 2017;15:746–748. [DOI] [PubMed] [Google Scholar]
- 22.Tauchmanova L, Alviggi C, Foresta C, et al. Cryptozoospermia with normal testicular function after allogeneic stem cell transplantation: a case report. Hum Reprod. 2007;22:495–499. [DOI] [PubMed] [Google Scholar]
- 23.Thomas LJ, Shim TN, Borysiewicz C, et al. Male genital lichen sclerosus in recipients of bone marrow transplants. Clin Exp Dermatol. 2016;41:495–497. [DOI] [PubMed] [Google Scholar]
- 24.Yared J, Gojo I, Akpek G. Glans penis involvement: an under-recognized manifestation of chronic GVHD. Bone Marrow Transplant. 2012;47:1006–1007. [DOI] [PubMed] [Google Scholar]
- 25.Andreini A, Zampieri N, Costantini C, et al. Chronic graft versus host disease is associated with erectile dysfunction in allogeneic hematopoietic stem cell transplant patients: a single-center experience. Leuk Lymphoma. 2018;59:2719–2722. [DOI] [PubMed] [Google Scholar]
- 26.Dyer G, Gilroy N, Bradford J, et al. A survey of fertility and sexual health following allogeneic haematopoietic stem cell transplantation in New South Wales, Australia. British journal of haematology. 2016;172:592–601. [DOI] [PubMed] [Google Scholar]
- 27.Mueller SM, Haeusermann P, Rovo A, et al. Genital chronic GVHD in men after hematopoietic stem cell transplantation: a single-center cross-sectional analysis of 155 patients. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1574–1580. [DOI] [PubMed] [Google Scholar]
- 28.Wong FL, Francisco L, Togawa K, et al. Longitudinal trajectory of sexual functioning after hematopoietic cell transplantation: impact of chronic graft-versus-host disease and total body irradiation. Blood. 2013;122:3973–3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2015;21:389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eeltink CM, Witte BI, Stringer J, et al. Health-care professionals’ perspective on discussing sexual issues in adult patients after haematopoietic cell transplantation. Bone Marrow Transplant. 2018;53:235–245. [DOI] [PubMed] [Google Scholar]
- 31.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greenfield DM, Walters SJ, Coleman RE, et al. Prevalence and consequences of androgen deficiency in young male cancer survivors in a controlled cross-sectional study. J Clin Endocrinol Metab. 2007;92:3476–3482. [DOI] [PubMed] [Google Scholar]
- 33.Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment: A Systematic Review. Endocr Rev. 2019;40:711–767. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee R, Kottaridis PD, McGarrigle HH, et al. Patterns of Leydig cell insufficiency in adult males following bone marrow transplantation for haematological malignancies. Bone Marrow Transplant. 2001;28:497–502. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee R, Andrews HO, McGarrigle HH, et al. Cavernosal arterial insufficiency is a major component of erectile dysfunction in some recipients of high-dose chemotherapy/chemo-radiotherapy for haematological malignancies. Bone Marrow Transplant. 2000;25:1185–1189. [DOI] [PubMed] [Google Scholar]
- 36.Greenfield DM, Boland E, Ezaydi Y, et al. Endocrine, metabolic, nutritional and body composition abnormalities are common in advanced intensively-treated (transplanted) multiple myeloma. Bone Marrow Transplant. 2014;49:907–912. [DOI] [PubMed] [Google Scholar]
- 37.Gundgurthi A, Garg MK, Nair V, et al. Endocrine complications after busulphan and cyclophosphamide based hematopoietic stem cell transplant: A single tertiary care centre experience. Indian J Endocrinol Metab. 2013;17:855–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schimmer AD, Ali V, Stewart AK, Imrie K, Keating A. Male sexual function after autologous blood or marrow transplantation. Biol Blood Marrow Transplant. 2001;7:279–283. [DOI] [PubMed] [Google Scholar]
- 39.Schneidewind L, Neumann T, Probst KA, Schmidt CA, Kruger W. Recovery from hypogonadism and male health in adult allogeneic stem cell transplantation. Eur J Haematol. 2018;100:584–591. [DOI] [PubMed] [Google Scholar]
- 40.Sirmatel P, Yilmaz H, Gündüz E. Non-malignant late effects in lymphoma patients treated with autologous hematopoietic stem cell transplantation. European Journal of Therapeutics. 2017;23:106–110. [Google Scholar]
- 41.Tauchmanova L, Selleri C, De Rosa G, et al. Endocrine disorders during the first year after autologous stem-cell transplant. Am J Med. 2005;118:664–670. [DOI] [PubMed] [Google Scholar]
- 42.Tauchmanova L, Selleri C, Rosa GD, et al. High prevalence of endocrine dysfunction in long-term survivors after allogeneic bone marrow transplantation for hematologic diseases. Cancer. 2002;95:1076–1084. [DOI] [PubMed] [Google Scholar]
- 43.Vaezi M, Gharib C, Souri M, Ghavamzadeh A. Late Complications in acute Leukemia patients following HSCT: A single center experience. Int J Hematol Oncol Stem Cell Res. 2016;10:1–6. [PMC free article] [PubMed] [Google Scholar]
- 44.Bhasin S, Brito JP, Cunningham GR, et al. Testosterone Therapy in Men With Hypogonadism: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2018;103:1715–1744. [DOI] [PubMed] [Google Scholar]
- 45.Taskinen M, Kananen K, Valimaki M, et al. Risk factors for reduced areal bone mineral density in young adults with stem cell transplantation in childhood. Pediatr Transplant. 2006;10:90–97. [DOI] [PubMed] [Google Scholar]
- 46.Anandi P, Jain NA, Tian X, et al. Factors influencing the late phase of recovery after bone mineral density loss in allogeneic stem cell transplantation survivors. Bone Marrow Transplant. 2016;51:1101–1106. [DOI] [PubMed] [Google Scholar]
- 47.Orio F, Muscogiuri G, Palomba S, et al. Endocrinopathies after allogeneic and autologous transplantation of hematopoietic stem cells. ScientificWorldJournal. 2014;2014:282147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Mewawalla P, Stratton P, et al. Sexual health in hematopoietic stem cell transplant recipients. Cancer. 2015;121:4124–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, et al. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta-analysis of randomized, placebo-controlled trials. J Clin Endocrinol Metab. 2018. [DOI] [PubMed] [Google Scholar]
- 50.Mulhall JP, Trost LW, Brannigan RE, et al. Evaluation and Management of Testosterone Deficiency: AUA Guideline. J Urol. 2018;200:423–432. [DOI] [PubMed] [Google Scholar]
- 51.Walsh JS, Marshall H, Smith IL, et al. Testosterone replacement in young male cancer survivors: A 6-month double-blind randomised placebo-controlled trial. PLoS Med. 2019;16:e1002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howell SJ, Radford JA, Adams JE, Smets EM, Warburton R, Shalet SM. Randomized placebo-controlled trial of testosterone replacement in men with mild Leydig cell insufficiency following cytotoxic chemotherapy. Clin Endocrinol (Oxf). 2001;55:315–324. [DOI] [PubMed] [Google Scholar]
- 53.Chatterjee R, Kottaridis PD, McGarrigle HH, Linch DC. Management of erectile dysfunction by combination therapy with testosterone and sildenafil in recipients of high-dose therapy for haematological malignancies. Bone Marrow Transplant. 2002;29:607–610. [DOI] [PubMed] [Google Scholar]
- 54.Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Valimaki MJ. Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab. 2005;90:3877–3885. [DOI] [PubMed] [Google Scholar]
- 55.Bar M, Ott SM, Lewiecki EM, et al. Bone Health Management After Hematopoietic Cell Transplantation: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26:1784–1802. [DOI] [PubMed] [Google Scholar]
- 56.Boquiren VM, Esplen MJ, Wong J, Toner B, Warner E, Malik N. Sexual functioning in breast cancer survivors experiencing body image disturbance. Psychooncology. 2016;25:66–76. [DOI] [PubMed] [Google Scholar]
- 57.Knoepp LR, Shippey SH, Chen CC, Cundiff GW, Derogatis LR, Handa VL. Sexual complaints, pelvic floor symptoms, and sexual distress in women over forty. J Sex Med. 2010;7:3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thygesen KH, Schjodt I, Jarden M. The impact of hematopoietic stem cell transplantation on sexuality: a systematic review of the literature. Bone Marrow Transplant. 2012;47:716–724. [DOI] [PubMed] [Google Scholar]
- 59.Syrjala KL, Langer SL, Abrams JR, Storer BE, Martin PJ. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–6606. [DOI] [PubMed] [Google Scholar]
- 60.Watson M, Buck G, Wheatley K, et al. Adverse impact of bone marrow transplantation on quality of life in acute myeloid leukaemia patients; analysis of the UK Medical Research Council AML 10 Trial. Eur J Cancer. 2004;40:971–978. [DOI] [PubMed] [Google Scholar]
- 61.Messerer D, Engel J, Hasford J, et al. Impact of different post-remission strategies on quality of life in patients with acute myeloid leukemia. Haematologica. 2008;93:826–833. [DOI] [PubMed] [Google Scholar]
- 62.Lee SJ, Fairclough D, Parsons SK, et al. Recovery after stem-cell transplantation for hematologic diseases. J Clin Oncol. 2001;19:242–252. [DOI] [PubMed] [Google Scholar]
- 63.Hjermstad MJ, Evensen SA, Kvaloy SO, Loge JH, Fayers PM, Kaasa S. The CARES-SF used for prospective assessment of health-related quality of life after stem cell transplantation. Psychooncology. 2003;12:803–813. [DOI] [PubMed] [Google Scholar]
- 64.Gruber U, Fegg M, Buchmann M, Kolb HJ, Hiddemann W. The long-term psychosocial effects of haematopoetic stem cell transplantation. Eur J Cancer Care (Engl). 2003;12:249–256. [PubMed] [Google Scholar]
- 65.Lee HG, Park EY, Kim HM, et al. Sexuality and quality of life after hematopoietic stem cell transplantation. Korean J Intern Med. 2002;17:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gifford G, Gilroy N, Dyer G, et al. The experience of survival following allogeneic haematopoietic stem cell transplantation in New South Wales, Australia. Bone Marrow Transplant. 2016;51:1361–1368. [DOI] [PubMed] [Google Scholar]
- 67.Bersvendsen HS, Haugnes HS, Dahl AA, et al. Sexual function in long-term male lymphoma survivors after high-dose therapy with autologous stem-cell transplantation. Bone Marrow Transplant. 2020;55:891–905. [DOI] [PubMed] [Google Scholar]
- 68.Syrjala KL, Schoemans H, Yi JC, et al. Sexual Functioning in Long-Term Survivors of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Socie G, Mary JY, Esperou H, et al. Health and functional status of adult recipients 1 year after allogeneic haematopoietic stem cell transplantation. British journal of haematology. 2001;113:194–201. [DOI] [PubMed] [Google Scholar]
- 70.Heinonen H, Volin L, Uutela A, Zevon M, Barrick C, Ruutu T. Gender-associated differences in the quality of life after allogeneic BMT. Bone Marrow Transplant. 2001;28:503–509. [DOI] [PubMed] [Google Scholar]
- 71.Noerskov KH, Schjodt I, Syrjala KL, Jarden M. Sexual function 1-year after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2016;51:833–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chiodi S, Spinelli S, Ravera G, et al. Quality of life in 244 recipients of allogeneic bone marrow transplantation. British journal of haematology. 2000;110:614–619. [DOI] [PubMed] [Google Scholar]
- 73.Humphreys CT, Tallman B, Altmaier EM, Barnette V. Sexual functioning in patients undergoing bone marrow transplantation: a longitudinal study. Bone Marrow Transplant. 2007;39:491–496. [DOI] [PubMed] [Google Scholar]
- 74.Mosher CE, DuHamel KN, Rini C, Corner G, Lam J, Redd WH. Quality of life concerns and depression among hematopoietic stem cell transplant survivors. Support Care Cancer. 2011;19:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Syrjala KL, Kurland BF, Abrams JR, Sanders JE, Heiman JR. Sexual function changes during the 5 years after high-dose treatment and hematopoietic cell transplantation for malignancy, with case-matched controls at 5 years. Blood. 2008;111:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Norskov KH, Schmidt M, Jarden M. Patients’ experience of sexuality 1-year after allogeneic Haematopoietic Stem Cell Transplantation. Eur J Oncol Nurs. 2015;19:419–426. [DOI] [PubMed] [Google Scholar]
- 77.Yoo KH, Kang D, Kim IR, et al. Satisfaction with sexual activity and sexual dysfunction in hematopoietic stem cell transplantation survivors and their partners: a couple study. Bone Marrow Transplant. 2018;53:967–976. [DOI] [PubMed] [Google Scholar]
- 78.Case P The impact of gender role on recovery from bone marrow transplantation. Illness Crisis & Loss. 2002;10:344–355. [Google Scholar]
- 79.Polomeni A, Lapusan S, Bompoint C, Rubio MT, Mohty M. The impact of allogeneic-hematopoietic stem cell transplantation on patients’ and close relatives’ quality of life and relationships. Eur J Oncol Nurs. 2016;21:248–256. [DOI] [PubMed] [Google Scholar]
- 80.Bishop MM, Beaumont JL, Hahn EA, et al. Late effects of cancer and hematopoietic stem-cell transplantation on spouses or partners compared with survivors and survivor-matched controls. J Clin Oncol. 2007;25:1403–1411. [DOI] [PubMed] [Google Scholar]
- 81.Langer S, Abrams J, Syrjala K. Caregiver and patient marital satisfaction and affect following hematopoietic stem cell transplantation: a prospective, longitudinal investigation. Psychooncology. 2003;12:239–253. [DOI] [PubMed] [Google Scholar]
- 82.Booker R, Walker L, Raffin Bouchal S. Sexuality after hematopoietic stem cell transplantation: A mixed methods study. Eur J Oncol Nurs. 2019;39:10–20. [DOI] [PubMed] [Google Scholar]
- 83.Kim IR, Jang SY, Shin HS, et al. Association between sexuality knowledge and sexual dysfunction in hematopoietic stem cell transplantation patients and their partners. Patient Educ Couns. 2020;103:1630–1636. [DOI] [PubMed] [Google Scholar]
- 84.Russell C, Harcourt D, Henderson L, Marks DI. Patients’ experiences of appearance changes following allogeneic bone marrow transplantation. Cancer Nurs. 2011;34:315–321. [DOI] [PubMed] [Google Scholar]
- 85.Carter J, Lacchetti C, Andersen BL, et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J Clin Oncol. 2018;36:492–511. [DOI] [PubMed] [Google Scholar]
- 86.El-Jawahri A, Fishman SR, Vanderklish J, et al. Pilot study of a multimodal intervention to enhance sexual function in survivors of hematopoietic stem cell transplantation. Cancer. 2018;124:2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Burnett AL, Nehra A, Breau RH, et al. Erectile Dysfunction: AUA Guideline. J Urol. 2018;200:633–641. [DOI] [PubMed] [Google Scholar]
- 88.Tichelli A, Rovo A. Fertility issues following hematopoietic stem cell transplantation. Expert Rev Hematol. 2013;6:375–388. [DOI] [PubMed] [Google Scholar]
- 89.Anserini P, Chiodi S, Spinelli S, et al. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447–451. [DOI] [PubMed] [Google Scholar]
- 90.Rovo A, Aljurf M, Chiodi S, et al. Ongoing graft-versus-host disease is a risk factor for azoospermia after allogeneic hematopoietic stem cell transplantation: a survey of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2013;98:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rovo A, Tichelli A, Passweg JR, et al. Spermatogenesis in long-term survivors after allogeneic hematopoietic stem cell transplantation is associated with age, time interval since transplantation, and apparently absence of chronic GvHD. Blood. 2006;108:1100–1105. [DOI] [PubMed] [Google Scholar]
- 92.Joshi S, Savani BN, Chow EJ, et al. Clinical guide to fertility preservation in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2014;49:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Logan S, Perz J, Ussher JM, Peate M, Anazodo A. Systematic review of fertility-related psychological distress in cancer patients: Informing on an improved model of care. Psychooncology. 2019;28:22–30. [DOI] [PubMed] [Google Scholar]
- 94.Ussher JM, Perz J, Australian C, Fertility Study T. Threat of biographical disruption: the gendered construction and experience of infertility following cancer for women and men. BMC Cancer. 2018;18:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borgmann-Staudt A, Rendtorff R, Reinmuth S, et al. Fertility after allogeneic haematopoietic stem cell transplantation in childhood and adolescence. Bone Marrow Transplant. 2012;47:271–276. [DOI] [PubMed] [Google Scholar]
- 96.Grigg AP, McLachlan R, Zajac J, Szer J. Reproductive status in long-term bone marrow transplant survivors receiving busulfan-cyclophosphamide (120 mg/kg). Bone Marrow Transplant. 2000;26:1089–1095. [DOI] [PubMed] [Google Scholar]
- 97.Lukusa AK, Vermylen C, Vanabelle B, et al. Bone marrow transplantation or hydroxyurea for sickle cell anemia: long-term effects on semen variables and hormone profiles. Pediatr Hematol Oncol. 2009;26:186–194. [DOI] [PubMed] [Google Scholar]
- 98.Panasiuk A, Nussey S, Veys P, et al. Gonadal function and fertility after stem cell transplantation in childhood: comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. British journal of haematology. 2015;170:719–726. [DOI] [PubMed] [Google Scholar]
- 99.Savani BN, Kozanas E, Shenoy A, Barrett AJ. Recovery of spermatogenesis after total-body irradiation. Blood. 2006;108:4292–4293; author reply 4293-4294. [DOI] [PubMed] [Google Scholar]
- 100.Wilhelmsson M, Vatanen A, Borgstrom B, et al. Adult testicular volume predicts spermatogenetic recovery after allogeneic HSCT in childhood and adolescence. Pediatr Blood Cancer. 2014;61:1094–1100. [DOI] [PubMed] [Google Scholar]
- 101.Mathiesen S, Sorensen K, Nielsen MM, et al. Male Gonadal Function after Allogeneic Hematopoietic Stem Cell Transplantation in Childhood: A Cross-Sectional, Population-Based Study. Biol Blood Marrow Transplant. 2020;26:1635–1645. [DOI] [PubMed] [Google Scholar]
- 102.Kerbauy MN, Mariano L, Seber A, Rocha V. The impact of low dose busulfan on gonodal function after allogeneic hematopoietic stem cell transplantation for aplastic anemia. Bone Marrow Transplant. 2020;55:1169–1171. [DOI] [PubMed] [Google Scholar]
- 103.Wagner AM, Beier K, Christen E, Hollander GA, Krenger W. Leydig cell injury as a consequence of an acute graft-versus-host reaction. Blood. 2005;105:2988–2990. [DOI] [PubMed] [Google Scholar]
- 104.Babb A, Farah N, Lyons C, et al. Uptake and outcome of assisted reproductive techniques in long-term survivors of SCT. Bone Marrow Transplant. 2012;47:568–573. [DOI] [PubMed] [Google Scholar]
- 105.Branvall E, Derolf AR, Johansson E, Hultcrantz M, Bergmark K, Bjorkholm M. Self-reported fertility in long-term survivors of acute myeloid leukemia. Ann Hematol. 2014;93:1491–1498. [DOI] [PubMed] [Google Scholar]
- 106.Carter A, Robison LL, Francisco L, et al. Prevalence of conception and pregnancy outcomes after hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Bone Marrow Transplant. 2006;37:1023–1029. [DOI] [PubMed] [Google Scholar]
- 107.Hammond C, Abrams JR, Syrjala KL. Fertility and risk factors for elevated infertility concern in 10-year hematopoietic cell transplant survivors and case-matched controls. J Clin Oncol. 2007;25:3511–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hayden PJ, Keogh F, Ni Conghaile M, et al. A single-centre assessment of long-term quality-of-life status after sibling allogeneic stem cell transplantation for chronic myeloid leukaemia in first chronic phase. Bone Marrow Transplant. 2004;34:545–556. [DOI] [PubMed] [Google Scholar]
- 109.Ishiguro H, Yasuda Y, Tomita Y, et al. Gonadal shielding to irradiation is effective in protecting testicular growth and function in long-term survivors of bone marrow transplantation during childhood or adolescence. Bone Marrow Transplant. 2007;39:483–490. [DOI] [PubMed] [Google Scholar]
- 110.Loren AW, Chow E, Jacobsohn DA, et al. Pregnancy after hematopoietic cell transplantation: a report from the late effects working committee of the Center for International Blood and Marrow Transplant Research (CIBMTR). Biol Blood Marrow Transplant. 2011;17:157–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rahal I, Galambrun C, Bertrand Y, et al. Late effects after hematopoietic stem cell transplantation for beta-thalassemia major: the French national experience. Haematologica. 2018;103:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salooja N, Szydlo RM, Socie G, et al. Pregnancy outcomes after peripheral blood or bone marrow transplantation: a retrospective survey. Lancet. 2001;358:271–276. [DOI] [PubMed] [Google Scholar]
- 113.Wilhelmsson M, Glosli H, Ifversen M, et al. Long-term health outcomes in survivors of childhood AML treated with allogeneic HSCT: a NOPHO-AML Study. Bone Marrow Transplant. 2019;54:726–736. [DOI] [PubMed] [Google Scholar]
- 114.Rovo A, Aljurf M, Chiodi S, et al. Paternity wishes in long-term survivors after allogeneic hematopoietic SCT. A study of the late effects working party of the EBMT. Bone Marrow Transplant. 2013;48:878–879. [DOI] [PubMed] [Google Scholar]
- 115.Balduzzi A, Dalle JH, Jahnukainen K, et al. Fertility preservation issues in pediatric hematopoietic stem cell transplantation: practical approaches from the consensus of the Pediatric Diseases Working Party of the EBMT and the International BFM Study Group. Bone Marrow Transplant. 2017;52:1406–1415. [DOI] [PubMed] [Google Scholar]
- 116.Nahata L, Cohen LE, Lehmann LE, Yu RN. Semen analysis in adolescent cancer patients prior to bone marrow transplantation: when is it too late for fertility preservation? Pediatr Blood Cancer. 2013;60:129–132. [DOI] [PubMed] [Google Scholar]
- 117.Schlegel PN, Sigman M, Collura B, et al. Diagnosis and treatment of infertility in men: AUA/ASRM guideline part II. Fertil Steril. 2020. [DOI] [PubMed] [Google Scholar]
- 118.Borgstrom B, Fridstrom M, Gustafsson B, Ljungman P, Rodriguez-Wallberg KA. A prospective study on the long-term outcome of prepubertal and pubertal boys undergoing testicular biopsy for fertility preservation prior to hematologic stem cell transplantation. Pediatr Blood Cancer. 2020;67:e28507. [DOI] [PubMed] [Google Scholar]
- 119.Bernaudin F, Dalle JH, Bories D, et al. Long-term event-free survival, chimerism and fertility outcomes in 234 patients with sickle-cell anemia younger than 30 years after myeloablative conditioning and matched-sibling transplantation in France. Haematologica. 2020;105:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Abdelaal O, Barber H, Atala A, Sadri-Ardekani H. Purging of malignant cell contamination prior to spermatogonia stem cell autotransplantation to preserve fertility: progress & prospects. Curr Opin Endocrinol Diabetes Obes. 2019;26:166–174. [DOI] [PubMed] [Google Scholar]
- 121.Hwee T, Bergen K, Leppke S, Silver A, Loren A. Hematopoietic Cell Transplantation and Utilization of Fertility Preservation Services. Biol Blood Marrow Transplant. 2019;25:989–994. [DOI] [PubMed] [Google Scholar]
- 122.Syrjala KL, Martin PJ, Lee SJ. Delivering care to long-term adult survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30:3746–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bilmon IA, Ashton LJ, Le Marsney RE, et al. Second cancer risk in adults receiving autologous haematopoietic SCT for cancer: a population-based cohort study. Bone Marrow Transplant. 2014;49:691–698. [DOI] [PubMed] [Google Scholar]
- 124.Gallagher G, Forrest DL. Second solid cancers after allogeneic hematopoietic stem cell transplantation. Cancer. 2007;109:84–92. [DOI] [PubMed] [Google Scholar]
- 125.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahindra A, Raval G, Mehta P, et al. New cancers after autotransplantations for multiple myeloma. Biol Blood Marrow Transplant. 2015;21:738–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baker KS, Leisenring WM, Goodman PJ, et al. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood. 2019;133:2790–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu PA, Stern RS, Huang V, et al. Reduced-Intensity Conditioning Regimens, Prior Chronic Lymphocytic Leukemia, and Graft-Versus-Host Disease Are Associated with Higher Rates of Skin Cancer after Allogeneic Hematopoietic Stem Cell Transplantation. J Invest Dermatol. 2019;139:591–599. [DOI] [PubMed] [Google Scholar]
- 129.Inamoto Y, Matsuda T, Tabuchi K, et al. Outcomes of patients who developed subsequent solid cancer after hematopoietic cell transplantation. Blood Adv. 2018;2:1901–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. [DOI] [PubMed] [Google Scholar]
- 131.Leisenring W, Friedman DL, Flowers ME, Schwartz JL, Deeg HJ. Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol. 2006;24:1119–1126. [DOI] [PubMed] [Google Scholar]
- 132.Tichelli A, Beohou E, Labopin M, et al. Evaluation of Second Solid Cancers After Hematopoietic Stem Cell Transplantation in European Patients. JAMA Oncol. 2019;5:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Atsuta Y, Suzuki R, Yamashita T, et al. Continuing increased risk of oral/esophageal cancer after allogeneic hematopoietic stem cell transplantation in adults in association with chronic graft-versus-host disease. Ann Oncol. 2014;25:435–441. [DOI] [PubMed] [Google Scholar]
- 134.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. [DOI] [PubMed] [Google Scholar]
- 135.Majhail NS, Brazauskas R, Rizzo JD, et al. Secondary solid cancers after allogeneic hematopoietic cell transplantation using busulfan-cyclophosphamide conditioning. Blood. 2011;117:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ringden O, Brazauskas R, Wang Z, et al. Second solid cancers after allogeneic hematopoietic cell transplantation using reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20:1777–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vajdic CM, Mayson E, Dodds AJ, et al. Second Cancer Risk and Late Mortality in Adult Australians Receiving Allogeneic Hematopoietic Stem Cell Transplantation: A Population-Based Cohort Study. Biol Blood Marrow Transplant. 2016;22:949–956. [DOI] [PubMed] [Google Scholar]
- 138.Yokota A, Ozawa S, Masanori T, et al. Secondary solid tumors after allogeneic hematopoietic SCT in Japan. Bone Marrow Transplant. 2012;47:95–100. [DOI] [PubMed] [Google Scholar]
- 139.Danner-Koptik KE, Majhail NS, Brazauskas R, et al. Second malignancies after autologous hematopoietic cell transplantation in children. Bone Marrow Transplant. 2013;48:363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shimoni A, Shem-Tov N, Chetrit A, et al. Secondary malignancies after allogeneic stem-cell transplantation in the era of reduced-intensity conditioning; the incidence is not reduced. Leukemia. 2013;27:829–835. [DOI] [PubMed] [Google Scholar]
- 141.Gutierrez-Pascual M, Vicente-Martin FJ, Lopez-Estebaranz JL. Lichen sclerosus and squamous cell carcinoma. Actas Dermosifiliogr. 2012;103:21–28. [DOI] [PubMed] [Google Scholar]
- 142.Snowden JA, McGrath E, Duarte RF, et al. JACIE accreditation for blood and marrow transplantation: past, present and future directions of an international model for healthcare quality improvement. Bone Marrow Transplant. 2017;52:1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


