Table 1.
Optimization of the formation of indole 3aa.
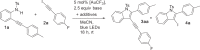 | |||||
|---|---|---|---|---|---|
| Entry | 1a:2a Ratio | Base | Additivesc | 3aa, yield (%)a | 4a, yield (%)a |
| 1b | 1:1.5 | K2CO3 | – | 8 | 10 |
| 2c | 1:1.5 | K2CO3 | 1 mol% [Ir] | 38 | 18 |
| 3 | 1:1.5 | K2CO3 | 10 mol% PhCOPh | 61 | 16 |
| 4 | 1:1.5 | K2CO3 | – | 64 | 16 |
| 5 | 1:1.1 | K2CO3 | – | 62 | 10 |
| 6 | 1:1.1 | Na2CO3 | – | 4 | 10 |
| 7 | 1:1.1 | Cs2CO3 | – | 10 | 6 |
| 8 | 1:1.1 | CaCO3 | – | 0 | 0 |
| 9 | 1:1.1 | K2CO3 | 1 equiv 18-C-6 | 14 | 16 |
aNMR yield using 1,3,5-trimethoxybenzene as internal standard; all reactions run on a 0.1 mmol except for entries 3 and 4 (0.3 mmol).
b5 mol% PPh3AuCl was used instead of 5 mol% [AuCF3].
c[Ir] = [Ir[dF(CF3)ppy]2(dtbbpy)PF6].
